Doxepin inhibits paclitaxel neuropathic pain
Info: 7715 words (31 pages) Dissertation
Published: 17th Feb 2022
Tagged: MedicinePharmacology
Doxepin reverses the expression and development of paclitaxel-induced neuropathic pain
Abstract
Anticancer drugs such as paclitaxel frequently produce peripheral neurotoxicity which leads to sensory function, mild paresthesia, dose reduction and discontinuation of treatment. This study was designed to evaluate the effect of acute and chronic doxepin administration on the development and expression of neuropathic pain during cancer chemotherapy.
Neuropathic pain was induced by paclitaxel (2 mg/kg, i.p., once daily from the first day to the 5th day) injection in the mice that resulted in mechanical and cold allodynia.
Acute doxepin injections (10 and 15 mg/kg i.p.) on the 11th day reduced tactile and cold hypersensitivity significantly compared to the control group.
When doxepin (2.5, 5 and 10 mg/kg i.p.) administered daily from the 1st day to the 5th day, cold and mechanical allodynia development significantly has been inhibited.
Besides doxepin (5 and 10 mg/kg i.p.) administration from the 6th day, once the model had been fully established, to 10th day significantly inhibit cold and mechanical allodynia expression.
Because of concerns related to decrease chemotherapy efficacy we also have evaluated paclitaxel cytotoxicity effect in combine with doxepin. Our results showed that doxepin even at high concentrations (1 and 10 µg/ml) didn’t interfere with cytotoxic effect of paclitaxel (0.05µg/ml).
These results indicate that doxepin, when administered during chemotherapy, can prevent the development and expression of paclitaxel induced neuropathic pain.
Key words: Paclitaxel, Neuropathic pain, Mice, Doxepin
Introduction
Anticancer drugs frequently produce peripheral neuropathy, which is clinically significant and may lead to dose reduction or discontinuation of treatment [1]. Paclitaxel is one of the most effective and frequently used chemotherapy for treatment of breast cancer [2]. However, paclitaxel produces peripheral neurotoxicity that leads to the sensory abnormalities and neuropathic pain during the treatment and it is even persisting after paclitaxel therapy. In some cases, neuropathic pain would impair patient’s quality of life and their daily activity [3].
Paclitaxel affects microtubules interrupts mitochondrial function and DNA synthesis. These effects on peripheral nerves may lead to spontaneous activity of fibers [4].
Nowadays the etiology and underlying mechanisms of such pain are poorly understood and the existing treatments including anticonvulsant agents, local anesthetics, and opioids are often ineffective [5, 6].
Chronic pain can cause depression in these patients. Tricyclic antidepressants (TCAs) is the most common treatment methods for chronic pain treatment [7-10].
Doxepin belongs to the TCA that inhibits serotonin and noradrenaline reuptake [11].
The analgesic property of doxepin like the other antidepressants has been reported (12). Topical application of doxepin produces analgesia. Mika et al have reported that doxepin and amitriptyline attenuated the symptoms of neuropathic pain in the mice subjected to chronic constriction injury (CCI), chronic administration of doxepin or amitriptyline also produced allodynia and hyperalgesia in naïve mice [13]. On the other hand Wrzosek et al have reported that doxepin was effective in reducing thermal hyperalgesia and mechanical allodynia [14].
Advantages of doxepin therapy include its lack of adverse interactions with prescription and non-prescription drugs taken and the high degree of safety seen in patients with concomitant cardiovascular and other physical disorders. Thus, doxepin appears to be an excellent choice in the long-term maintenance outpatient treatment of chronic depression [15].
Research has also suggested an interaction between antidepressants and opioid receptors. Activation of the endogenous opioid mechanisms by serotonergic and/or noradrenergic pathways is believed to be involved in the antinociceptive effect of antidepressants [16, 17].
Chronic antidepressant administration can modify opioid receptor densities and increase endogenous opioid levels in certain brain regions [18].
The anti-nociceptive effects of doxepin and it’s mechanisms of action in chemotherapy induced neuropathic pain have not been well understood.
This study was designed to determine the effect of acute and chronic doxepin administration and its related mechanisms in alleviating symptoms of paclitaxel-induced neuropathic pain in mice.
To identify the role of opioid system in the anti-nociceptive effects of doxepin in the neuropathic pain, we examined the effect of naloxone (an opioid receptor antagonist) on the anti-nociceptive effects of doxepin in paclitaxel induced neuropathic pain. Also because of concerns related to decrease chemotherapy efficacy we also have evaluated paclitaxel cytotoxicity effect in combine with doxepin.
2. Materials and Methods
2.1. Animals
NMRI male mice weighing 20-30 g were used. They were housed under a 12-hour light/dark cycle and free access to food and water. The animals were randomly assigned to different treatment groups (n=6-8 in each group). All studies were conducted in accordance with the guidelines for the care of laboratory animals of Ethics Committee of Isfahan University of Medical Sciences and followed the European Commission Directive (86/609/EEC) for animal experiments.
2.2. Chemicals
Paclitaxel was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Paclitaxel was dissolved in ethanol-cremophor-saline (5:5:90, v/v/v). Doxepin was purchased from Razak Laboratory (Tehran, Iran). Naloxone was obtained from Touliddarou (Tehran, Iran). They all dissolved in normal saline 0.9%.
2.3. General procedures for drug treatments
To induce the neuropathic pain, paclitaxel (2 mg/kg) intra peritoneally (i.p) was injected on the 1st day and given daily for 4 additional days with cumulative dose of 10 mg/kg (19). Sham group received only vehicle.
To assess the effects of doxepin on the development (chronic effect) of neuropathic pain, Doxepin (2.5, 5 and 10 mg/kg i.p.) were administered daily from the 1st day to the 5th day in the different groups of mice. Also doxepin was injected (2.5, 5 and 10 mg/kg) once daily from the 6th day until the 10th day. Mechanical and cold allodynia were assessed on the 11th day after the first paclitaxel injections [20].
To determine the effect of doxepin on the expression (acute effect) of paclitaxel-induced neuropathic pain, it was injected 30 min before pain assessment on the 11thday.
2.4. Behavioral tests of neuropathic pain
Animals were placed on top of an aluminum mesh table. They were allowed to adapt for 15 minute. For assessment the cold allodynia, acetone drop was applied via a needle to the plantar surface of each hind paw for three times with 30 seconds interval. The time spent for licking the paw was recorded. The frequency of licking was calculated and expressed as a percentage with the following formula (number of trial attending the hind-paw /total number of trial) × 100. For assessment the mechanical allodynia, von Frey filaments (Steeling, Wood Dale, IL, USA) ranging from 0.16 to 6 g were used as previously described [17]. Bending force was applied to the plantar skin of the right hind paw, and each application was held for 6 seconds, using the up–down method to determine threshold sensitivity.
2.5. Evaluation of the role of opioid receptors
To identify the role of opioid system in the anti-nociceptive effects of doxepin, animals were treated by naloxone (1 mg/kg i.p.) on the 11thday. Naloxone was injected 10 min before behavioral tests in different groups of mice. In these cases doxepin (15 mg/kg i.p.) was administrated form the 6thday to the 10th day.
2.6. In vitro cytotoxic activity
Human breast cancer cell lines, MCF-7 ( ER+ ) and MDA-MB231 (estrogen receptor negative [ER−]) were purchased from the National Cell Bank of Pasteur Institute of Iran. Cell lines were maintained at 37°C and 5% CO2. The different cell populations were cultured in RPMI (Biosera, France) containing 10% FBS (Gibco, USA).
In vitro cytotoxicity assay was initiated by separately plating (180 μl) of two cell line (5×104 cells/ ml of media) in 96-well micro plates and incubating for 24 h (37°C, air humidified 5% CO2). After 24h, doxepin and paclitaxel were added together to the 96-well micro plate to obtain 1 and 10 μg/ml and 0.05 μg/ml concentrations, respectively. They also added to the plates separately with the mentioned doses. Wells containing 180 μl of the cell suspension and 20 μl of DMSO (1%) were considered as negative control while the blank wells contained only 200 μl of the RPMI medium. The micro-plates were further incubated for 48 h. Each well was then treated with 20 μl of MTT solution for 3 h. Afterwards, the media in each well was replaced with 200 μl DMSO to dissolve the blue insoluble formazan crystals. The metabolic activity in each well was determined by a rapid colorimetric assay using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). Plates were read by using an enzyme-linked immunosorbent assay (ELISA) plate reader at 540 nm. The cell viability was determined by the following formula 1 and was compared with untreated control [21].
formula 1:
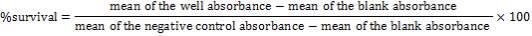
2.7. Data analysis
Data are presented as mean ± standard deviation. Data were compared by one-way analysis of variance (ANOVA) followed by Fisher LSD post-hoc test for multiple comparisons. Time-course analysis of behavioral data was compared by repeated measures ANOVA for each experimental group. Two-way analysis of variance (ANOVA) were used to compare the antagonistic effect of naloxone.
3. Results
3.1. Time-course of paclitaxel-induced cold and mechanical allodynia
As shown in Figure1A and 2A mice, which were treated with paclitaxel, had become much more sensitive to acetone application in paw withdrawal frequency (F4,29=12.67) and duration of paw licking (F4,29=8.071) in comparison with control group (p<0.001). Paclitaxel injection led to a significant decrease of paw withdrawal threshold in comparison with control group as shown in Figure 3 (F4,29=4.521) (p<0.01).
3.2. Effect of doxepin on the expression of cold allodynia
Acute administration of doxepin (10 and 15 mg/kg i.p.) 30 minute before acetone test on the 11thday significantly blocked paw withdrawal frequency (F4,29=12.67) and duration of paw licking (F4,29=8.071) (Figure 1A and 2A) (p<0.001).
3.3. Effect of doxepin on the expression of mechanical allodynia
As shown in Figure 3A, the statistically significant difference was observed in the animals treated with acute administration of doxepin (10 and 15 mg/kg i.p.) on the 11th day in comparison with control group (F4,29=4.521) (p<0.01).
3.4. Effect of doxepin on the development of cold allodynia
Chronic treatment with doxepin (5 and 10 mg/kg) from the 1st day until the 6th day produced significant anti nociceptive effects against paw withdrawal frequency (F4,29=15.44) and duration of paw licking (F4,29=14.423) compare to control group (Fig. 1B and 2B) (p<0.001). Also administration of doxepin (5 and 10 mg/kg i.p.) from the 6th day until the 10th day reduced paw withdrawal frequency (F4,29=6.303) and duration of paw licking (F4,29=6.785) (Fig.1C and 2C) (p<0.001).
3.5. Effect of doxepin on the development of mechanical allodynia
Chronic administration of doxepin (5 and 10 mg/kg i.p.) from 1st day until the 6th day (F4,29=10.515) and also from 6th day (F4,29=6.776), after establishment of neuropathic pain to the 10th day noticeably reduced paw withdrawal threshold compare to control group (Fig. 3B and C) (p<0.01).
3.6. Effects of naloxone on doxepin-induced effects
Naloxone (1 mg/kg i.p.) did not induce any significant change in the nociceptive thresholds.
Figures 4A, B and C show the effect of naloxone (1 mg/kg i.p.) on the anti-nociceptive effect of doxepin injection (15 mg/kg i.p.). Naloxone could not change anti allodynic effects of doxepin on the paw withdrawal frequency (F3,21=16.356) (Fig 4A), duration of paw licking (F3,21=5.954) (Fig. 4B) and mechanical allodynia (F3,21=5.213) (Fig. 4C).
3.7. Cytotoxicity effect of doxepin
The cytotoxicity effect of doxepin on MCF-7 and MDA cell lines was evaluated through micro-culture tetrazolium assay (MTT). Results of the cytotoxicity evaluation against MCF-7 and MDA cell lines are shown in figure 5. Doxepin (1 and 10 µg/ml) exhibited no significant activity against MCF-7 and MDA cell lines. On the contrary paclitaxel (0.05 µg/ml) exhibited significant activity against both cell lines. Also doxepin did not reverse the cytotoxicity effect of paclitaxel (Figures 5A and B).
4. Discussion
Chemotherapy-induced peripheral neuropathy is a relatively common and serious consequence of cancer treatment and, since it is often the main reason for reduction or discontinuation of therapy, it may limit the application of lifesaving agents. We found that systemic administration of doxepin inhibited paclitaxel-induced neuropathic pain.
Considering our results, acute doxepin (10 and 15 mg/kg i.p.) administration inhibited the expression of paclitaxel-induced mechanical and cold allodynia in mice. The repeated co administration of doxepin (5 and 10 mg/kg i.p.) and paclitaxel prevented the development of both kinds of allodynia. When doxepin (5 and 10 mg/kg i.p.) administration was initiated at the 6th day, once the model had been fully established, and given daily for 4 additional days, mechanical and cold allodynia significantly have been blocked. Results from doxepin cytotoxicity evaluation indicated lack of cytotoxic property and didn’t interfere with cytotoxic effect of paclitaxel.
Note that considerable adverse effects of doxepin such as dizziness, drowsiness or ataxia were not observed. This result is consistent with previous studies that suggested doxepin did not decrease motor performance at low doses (i.e. <30 mg/kg) [13, 22].
Since pain is a complex neurobiological phenomenon with a diversity of neurochemical factors contributing to the both peripheral and central pain-signaling mechanisms its possible that various mechanisms are involved in the anti-nociceptive effect of doxepin.
The earliest focus, with regard to the mechanism of action, was the ability of doxepin to inhibit biogenic amine reuptake [23]. Doxepin inhibits the reuptake of serotonin (5-HT) and, to a lesser extent, noradrenaline (NE).
Given that both 5-HT and NE are involved in the nociception, it is possible that antidepressants that act by modulating these neurotransmitters produced antinociceptive effects. Although studies suggest that antidepressants that target both 5-HT and NE neurotransmission are more effective than serotonergic agents in relieving various types of chronic pain.
Research has also suggested an interaction between antidepressants and opioid receptors. Activation of the endogenous opioid mechanisms by serotonergic and/or noradrenergic pathways is believed to be involved in the antinociceptive effect of antidepressants [16, 17].
Chronic antidepressant administration can modify opioid receptor densities and increase endogenous opioid levels in certain brain regions [18]. It appears that antidepressants may interact both directly and indirectly with endogenous opioid systems to produce analgesia [24].
For example, a study evaluating venlafaxine and mirtazapine found that both agents significantly potentiated antinociceptive effects through interactions with multiple opioid receptors. Other investigations have demonstrated inhibitory effects of naloxone an opioid antagonist on the analgesic effects of antidepressants [8]. Additional data suggest that dual-acting antidepressants (e.g., TCAs) may produce analgesic effects as a result of both direct and indirect interaction with opioid systems [25].
In the present study, we have investigated whether opioid-related mechanisms contribute to the antinociceptive effects of doxepin in paclitaxel induced neuropathic pain. Our results showed that naloxone as an opioid antagonist could not reverse the antinociceptive effects of doxepin.
These results demonstrate that doxepin, which is TCAs, differs from the other tricyclic antidepressants in it antinociception mechanisms.
Interestingly it’ apparent that TCAs exhibit diverse pharmacological properties and this may account for differing specific pharmacological profiles between agents.
The differential involvement of myelinated and unmyelinated peripheral nerve fibers in the pathogenesis of each sign of pain has been reported. Mechanical allodynia is evoked by inputs from myelinated fibers (mainly Aβ-fibers, but also Aβ-fibers), whereas heat hyperalgesia is evoked mainly by unmyelinated C-fibers, and both A – and C-fibers are recruited to mediate cold allodynia [26].
It is well known that voltage gated Na+ channels play a critical role in neuronal function and in the development of chemotherapy-induced peripheral neurotoxicity (CINP) [27]. Doxepin inhibits sodium channels which may participate in it’s antinociception effects [28]. However further studies are needed to validate this hypothesis.
5. Conclusions
These results indicate that doxepin, when administered during chemotherapy or ever after that can prevent the expression and development of neuropathic pain. This effect is not inhibited by naloxone which, interestingly, distinguishes doxepin from the other TCAs. Further investigations are required to clarify the possible mechanisms of doxepin in attenuation of neuropathic pain.
Also we have showed that doxepin didn’t interfere with cytotoxic effect of paclitaxel. These results suggest a potential use of doxepin in the management of neuropathic pain during chemotherapy with paclitaxel. However, further pre-clinical and clinical studies are needed to validate this effect and establish the effective doses of doxepin in human, when prescribe for pain.
6. References
[1] Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. (2014) 155 2461-70.
[2] Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, et al. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain (2013) 154 2432-40.
[3] Bráz JM, Wang X,Guan Z, Rubenstein JL, Basbaum AI. Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain. (2015) 156 1084-91.
[4] Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. (2008) 9 505-17.
[5] Tsuda M. Mechanisms underlying the pathogenesis of neuropathic pain revealing by the role of glial cells. Nihon ShinkeiSeishinYakurigaku Zasshi. (2015) 35 1-4.
[6] Carozzi VA, Canta A, Chiorazzi A.Chemotherapy-inducedperipheral neuropathy: What do we know about mechanisms.Neurosci Lett. (2015) 596 90-107.
[7] Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of venlafaxine in a rat model of peripheral neuropathy: role of alpha2-adrenergic receptors. Eur J Pharmacol (2014) 738 230-6.
[8] Abed A, Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A. Venlafaxine Attenuates Heat Hyperalgesia Independent of Adenosine or Opioid System in a Rat Model of Peripheral Neuropathy. Iran J Pharm Res (2015) 14 843-50.
[9] Hajhashemi V, Minaiyan M, Banafshe HR, Mesdaghinia A, Abed A. The anti-inflammatory effects of venlafaxine in the rat model of carrageenan-induced paw edema. Iran J Basic Med Sci (2015) 18 654-8.
[10] BanafsheHR, HajhashemiV, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of maprotiline in a rat model of peripheral neuropathic pain: possible involvement of opioid system. Iran J Basic Med Sci. (2015) 18 752-757.
[11] Yeung WF, Chung KF, Yung KP, Ng TH. Doxepin for insomnia: a systematic review of randomized placebo-controlled trials. Sleep Med Rev. (2015) 19 75-83.
[12] Wordliczek J, Banach M, Labuz D, Przewlocka B. Intrathecal administration of doxepin attenuated development of formalin-induced pain in rats. J Neural Transm. (1977) 112 1321-9.
[13] Mika J, Jurga AM, Starnowska J, Wasylewski M, Rojewska E, Makuch W. Effects of chronic doxepin and amitriptyline administration in naïve mice and in neuropathic pain mice model. Neuroscience. (2015) 294 38-50.
[14] Wrzosek A, Obara I, Wordliczek J, Przewlocka B. Efficacy of tramadol in combination with doxepin or venlafaxine in inhibition of nociceptive process in the rat model of neuropathic pain: an isobolographic analysis. J Physiol Pharmacol. (2009) 60 71-8.
[15] Ayd FJ Jr. Long-term treatment of chronic depression: 15-year experience with doxepin HCl. J Clin Psychiatry. (1984) 45 39-46.
[16] Duman EN, Kesim M, Kadioglu M, Yaris E, Kalyoncu NI, Erciyes N. Possible involvement of opioidergic and serotonergic mechanisms in antinociceptive effect of paroxetine in acute pain. J Pharmacol Sci. (2004) 94 161-5.
[17] Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. Gabapentin enhances anti-nociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci. (2014) 17 753-9.
[18] Isenberg KE, Cicero TJ. Possible involvement of opiate receptors in the pharmacological profiles of antidepressant compounds. Eur J Pharmacol. (1984) 103 57-63.
[19] Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. Eur J Pharmacol. (2001) 429 1-11.
[20] Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian AA, Abed A, Rafieian-Kopaei M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. (2015) 3 1-6
[21] Freshney RI.Culture of animal cells: A manual of basic technique. 4rd ed. United State: Wiley-Liss press (1994) p. 1-5, 9-15, 181-184, 309.
[22] Sun XY, Zhang L, Wei CX, Piao HR, Quan ZS. Characterization of the anticonvulsant activity of doxepin in various experimental seizure models in mice. Pharmacol Rep (2009) 61 245-51.
[23] Marks DM, Shah MJ, Patkar AA, Masand PS, Park GY, Pae CU. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuro pharmacol . (2009) 7 331-6.
[24] Hamon M, Gozlan H, Bourgoin S, Benoliel JJ, Mauborgne A, Taquet H, et al. Opioid receptors and neuropeptides in the CNS in rats treated chronically with amoxapine or amitriptyline. Neuropharmacology. (1987) 26 531-9.
[25] Olianas MC, Dedoni S, Onali P. The atypical antidepressant mianserin exhibits agonist activity at κ-opioid receptors. Br J Pharmacol. (2012) 167 1329-41.
[26] Hsieh MT, Donaldson LF, Lumb BM. Differential contributions of A- and C-nociceptors to primary and secondary inflammatory hypersensitivity in the rat. Pain (2015) 156 1074-83.
[27] Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens, JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. (2008) 137 520-31.
[28] Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C. Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. (1998) 284 208-14.
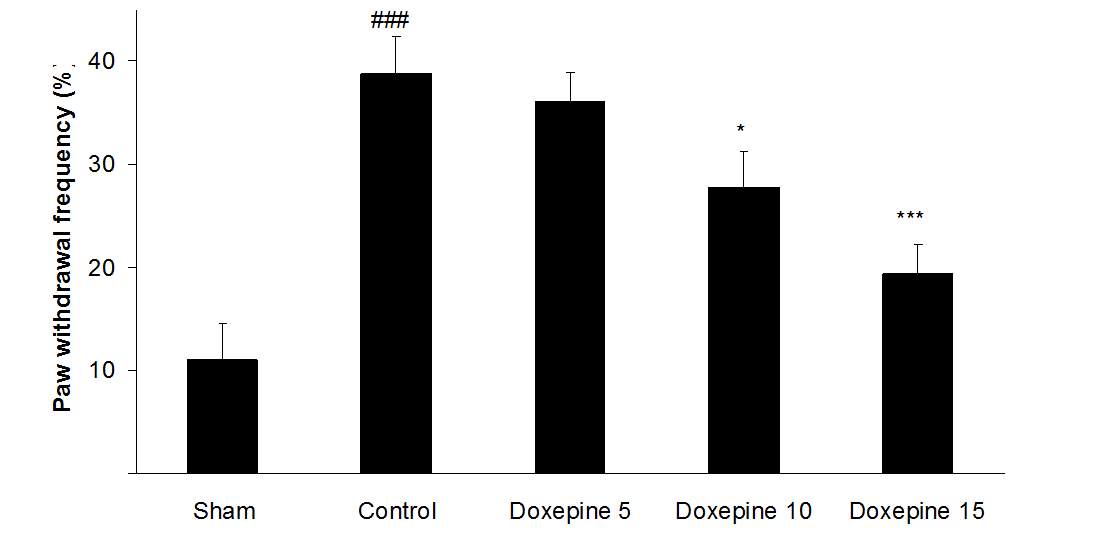
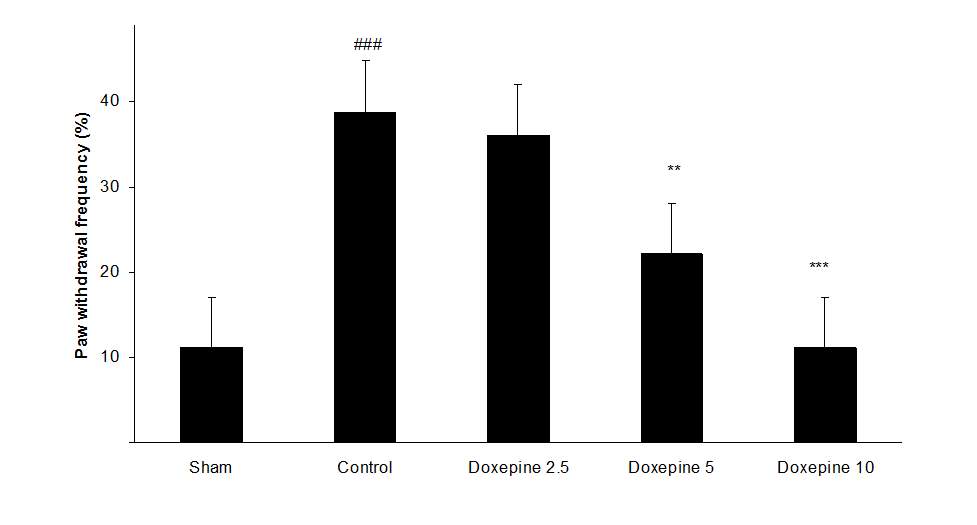
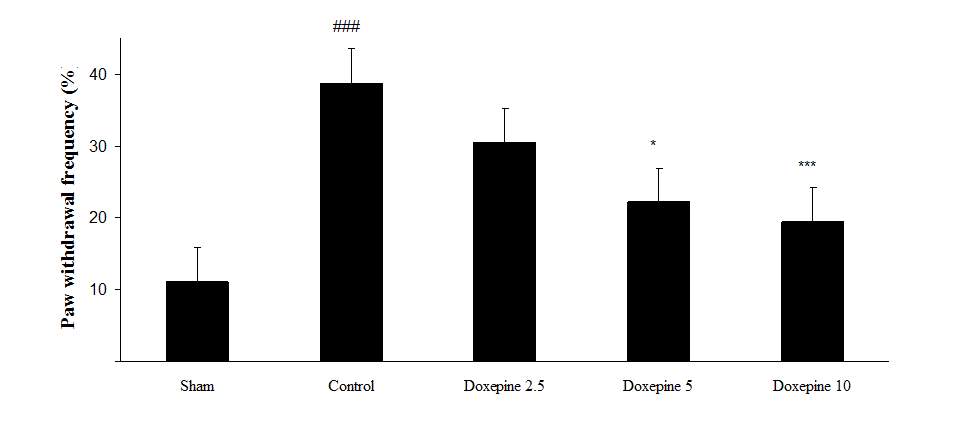
Fig .1 Paw withdrawal frequency. (A), Effect of acute treatment with doxepin (5, 10 and 15 mg/kg i.p.) on the paw withdrawal frequency. (B), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 1st day to the 6th day. (C), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 6th day to the 10th day. The results are expressed as Mean ± S.E.M., ###P<0.001 versus sham group, *P<0.05, **P< 0.01 and ***P<0.001 versus control (mice were treated with paclitaxel 2mg/kg i.p.) group, n=6 in all groups.
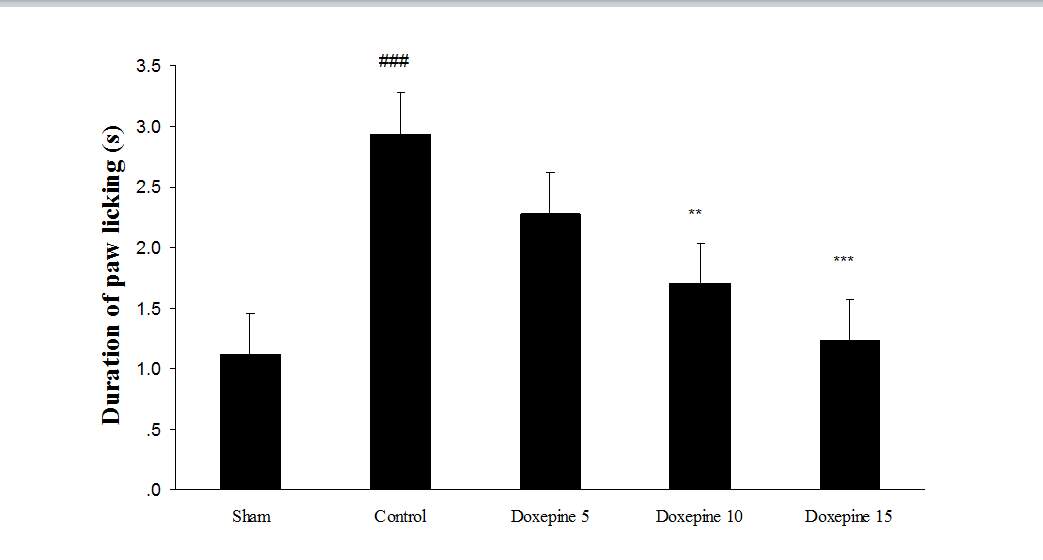
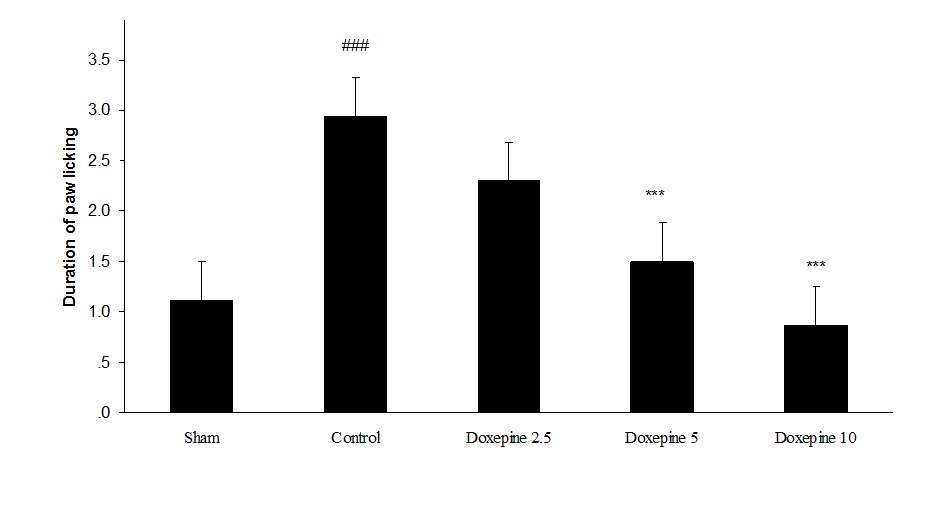
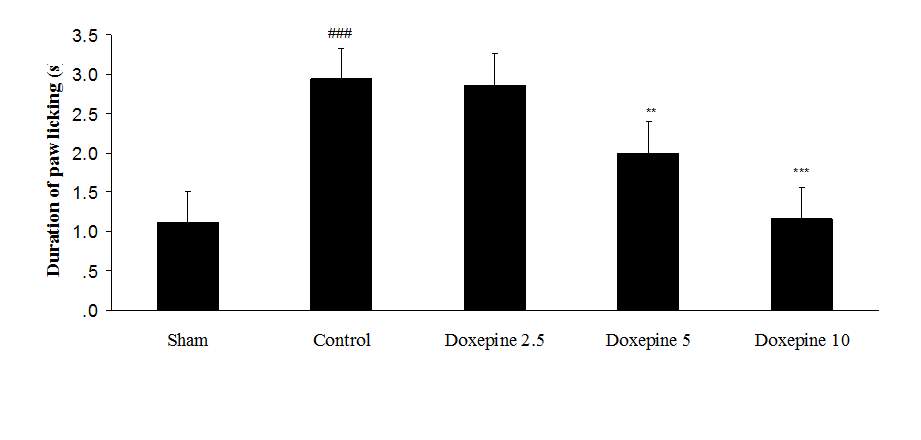
Fig. 2 Duration of paw licking.(A), Effect of acute treatment with doxepin (5, 10 and 15 mg/kg i.p.) on the duration of paw licking. (B), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 1st day to the 6th day. (C), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 6th day to the 10th day. The results are expressed as Mean ± S.E.M., ###P<0.001 versus sham group, **P< 0.01 and ***P<0.001 versus control (mice were treated with paclitaxel 2mg/kg i.p.) group, n=6 in all groups.
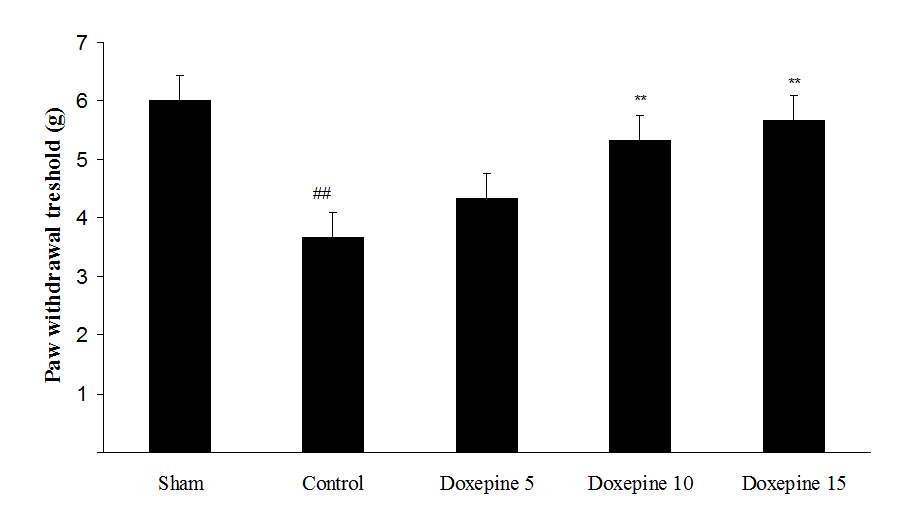
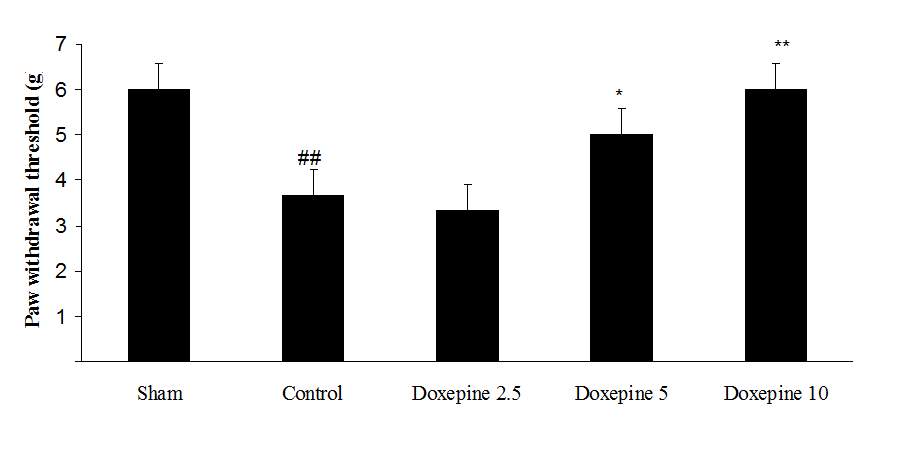
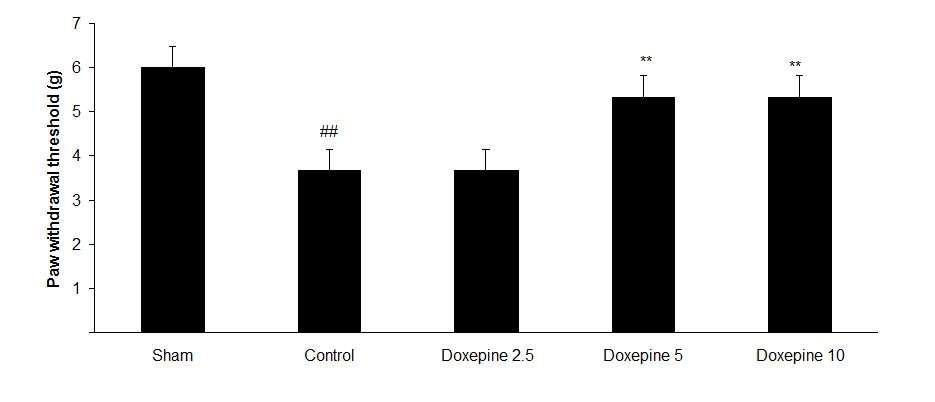
Fig3. Mechanical allodynia, (A) Effect of acute treatment with doxepin (5, 10 and 15 mg/kg i.p.) on the paw withdrawal threshold. (B), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 1st day to the 6th day. (C), Effect of chronic treatment with doxepin (2.5, 5 and 10 mg/kg i.p.) from 6th day to the 10th day. The results are expressed as Mean ± S.E.M., ##P<0.01 versus sham group, *P<0.05, **P< 0.01 and ***P<0.001 versus control (mice were treated with paclitaxel 2mg/kg i.p.) group, n=6 in all groups.
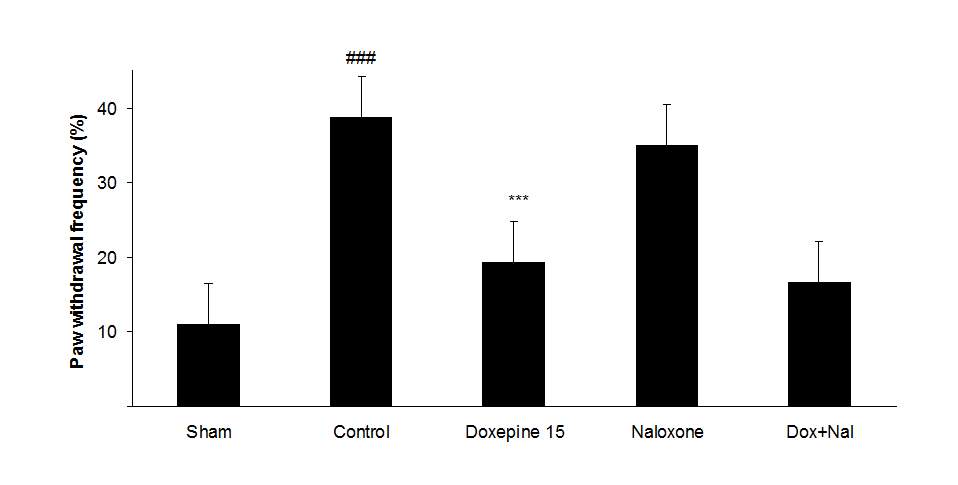
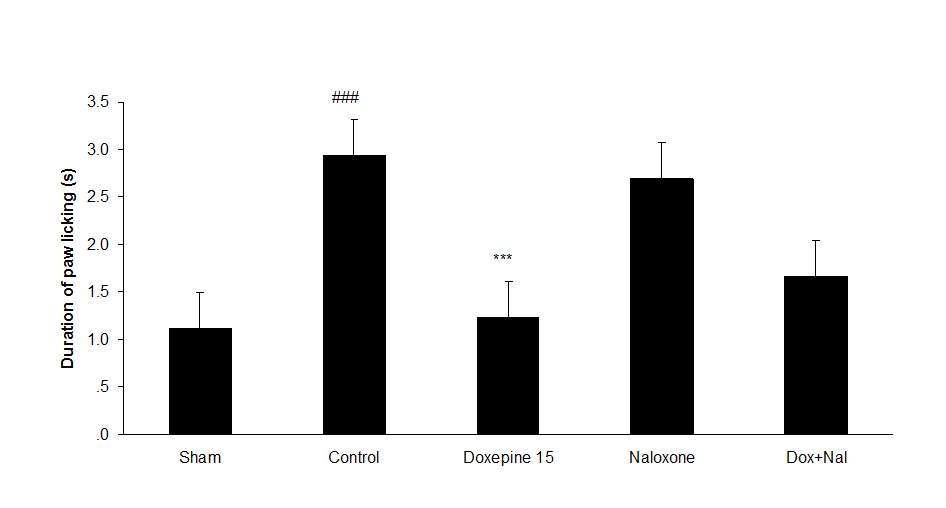
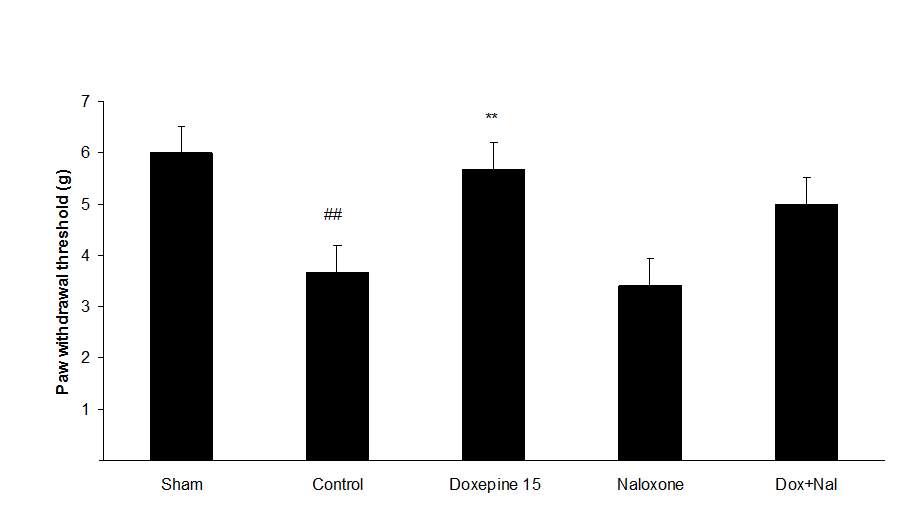
Fig.4. (A) The effect of naloxone (1mg/kg i.p.) on the antiallodynic effect of acute treatment with doxepin (15 mg/kg i.p.) on the paw withdrawal frequency. (B) The effect of naloxone (1mg/kg i.p.) on the antiallodynic effect of acute treatment with doxepin (15 mg/kg i.p.) on the duration of paw licking. (C) The effect of naloxone (1mg/kg i.p.) on the anti allodynic effect of acute treatment with doxepin (15 mg/kg i.p.) on the paw withdrawal threshold. The results are expressed as Mean ± S.E.M., n=6 in all groups.##P<0.01, ###P<0.001 versus sham group,**P<0.01, ***P<0.001 versus control (mice were treated with paclitaxel 2mg/kg i.p.) group.
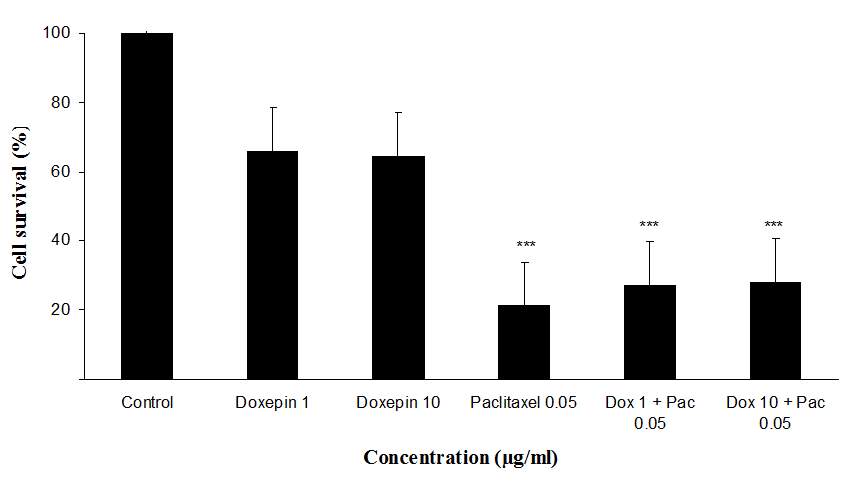
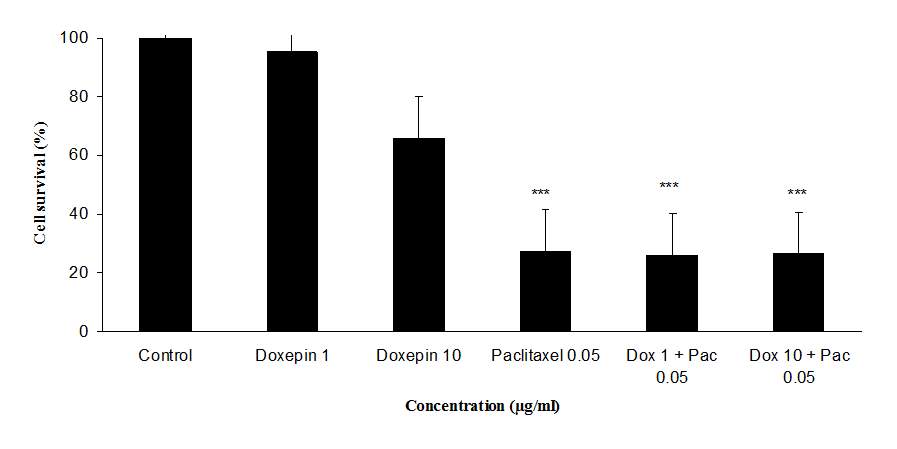
Fig 5.Cytotoxicity effect of doxepin, (A) Evaluation the cytotoxicity effect of doxepin (1 and 10 µg/ml) alone and in combination with paclitaxel (0.05 µg/ml) on MDA cell lines., (B)Evaluationthe cytotoxicity effect of doxepin (1 and 10 µg/ml) alone and in combination with paclitaxel (0.05 µg/ml) on MCF-7 cell lines, ***P<0.001 versus control (vehicle treated cells) group, Dox 1+ Pac 0.05 (cells were treated with doxepin 1µg/ml in combination with paclitaxel 0.05µg/ml), Dox 10 + Pac 0.05 (cells were treated with doxepin 10µg/ml in combination with paclitaxel 0.05µg/ml).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




