Solid Lipid Nanoparticles in Cancer Treatment
Info: 10719 words (43 pages) Dissertation
Published: 24th Feb 2022
Tagged: CancerBiomedical Science
Abstract
The focus of drug discovery has switched from the concept of the ‘magic bullet’ drugs which target specific cells to the concept of ‘magic wands’ which refers to the mode of drug delivery. Drug delivery systems offer an opportunity of enhanced therapeutic effect of anticancer agents by either increasing drug concentration to tumour sites and/or reducing exposure to normal tissues. Among the variety of delivery systems that have been developed, solid lipid nanoparticles (SLNs) have lead advancements in the field due to their unique capability to carry a wide variety of substances, structural versatility and biocompatibility of their components. There are many different drug delivery systems currently being developed such as polymeric and lipid micelles and nanoparticles. However, SLNs is one of the Nanoformulations that have recently been used further from scientists due to many advantageous, such as controlled drug release, non-biotoxicity of the carrier, increased bioavailability of drug and lower overall cost. This paper covers the techniques for the production of SLN, drug incorporation, loading capacity and drug release mechanisms and also the potential of SLNs in cancer treatment as an anti-cancer drug delivery vehicle.
Keywords: Cancer; Drug Delivery; Lipids; Nanoformulations; Nanoparticles; SLNs.
1. Introduction
The past decade has witnessed a remarkable growth in the field of nanotechnology as it offers a great scope to deliver small molecular drugs and macromolecules which include peptides, proteins and genes at the targeted site. The nanometre size range is proved to have several advantages as drug delivery systems. The application of nanotechnology in the fields of medicine and drug delivery in particular is on rise and it is anticipated to bring remarkable changes in the diagnosis and treatment of various diseases.
Novel drug delivery systems offer the possibility of enhanced therapeutic effect of anticancer agents by either increasing drug concentration to tumour sites and/or reducing exposure to normal tissues sites. Lipid drug delivery systems currently serve as useful tools for delivery of active anticancer agents (for example lipid formulations with doxorubicin or paclitaxel). Among the variety of delivery systems that have been developed, solid lipid nanoparticles (SLNs) have created the most excitement due to their unique properties such as ability to carry a wide variety of substances, structural versatility and biocompatibility of their components. Moreover, lipid based drug delivery systems are able to modify the pharmacokinetics and pharmacodynamics of cancer cells, allowing an increase in the localised concentration of drug released into the target cell without losing drug properties, thus reducing exposure of normal cells to drug molecules.
SLNs are colloidal deliver systems made from solid (under room temperature) lipid, with a diameter between 50 to 500 nm. SLNs, unlike polymeric nanoparticles, can be produced by a variety of different conveniently methods, such as high-pressure homogenisation. The obtained SLNs can be stabilised by surfactant such as lecithin, Tween 80, Pluronic 68 or by the combination of different surfactants [1]. SLNs are invented in early 90s, and it’s the latest development of lipid based colloidal delivery system after nanoemulsions (made from lipids that are liquid under room temperature). It quickly generated broad public attention within few years. The first safe emulsion for parental nutrition delivery was invented by Wretlind in 1961 [2], which marks the beginning of emulsion as colloidal delivery for lipophilic drugs, and after years of research some of them successively commercialised. By applying oil-in-water (O/W) emulsions were able to reduce injection dosage and thereby minimize side effect; o/w emulsion was designed and applied for drug delivery. Products such as Diazemuls and Diazepam-Lipuro were developed and placed into the market. Despite of the advantages, major limitations of these emulsions are also clear, such as the poor physical stability of drug containing emulsion. Moreover, the encapsulation of drugs is able to cause agglomeration, drug expulsion and poor ability to offer protection to liable compounds. Another lipid based carrier developed earlier is liposome, which normally composed of phospholipid. It is invented as early as 1965 with focus on cosmetic market. After one decade of research, several products such as lung surfactant for pulmonary instillation were put into the market. However, the total number of successful product is rather limited when compared to emulsion. Major obstacle is lack of commercially available production method. In another word, liposome product is only feasible in lab scale. Polymeric nanoparticles research has been under intensive research for 50 years. This delivery system is well commercialised like lipid based delivery system, however has encountered similar issues with liposomes; polymeric particles are difficult to be produced in large quantity, and also is been reported that polymeric particles have poor tolerability.
2. Solid Lipid Nanoparticles (SLNs)
SLNs were first introduced in 1991, and they represent alternative carrier systems to other traditional colloidal carriers namely O/W emulsions, liposomes, micro-particles and polymeric nanoparticles. SLN consists of spherical lipid particles in the nano-meter size range (Figure 1).
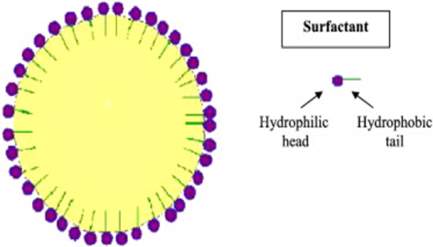
Figure 1. A diagrammatic representation of SLN.
SLN is used for the controlled and targeted delivery of drugs. The drugs are part of the solid lipid matrix. SLN is stabilised by a surfactant layer, which may consist of a single surfactant, but is normally composed of a mixture of surfactants. Generally, a solid lipid that is used in such delivery systems melts at temperatures exceeding body temperature (37 °C). Some of these lipids are fatty acids, steroids, waxes, triglycerides, acyl-glycerols or a combination thereof. Different classes and ratios of emulsifiers have been successfully utilised to stabilise SLNs. Some of the most common emulsifiers are lecithin, bile salts such as sodium taurocholate, non-ionic emulsifiers such as ethylene oxide/propylene oxide copolymers, sorbitan esters, fatty acid ehoxylates etc. SLN is claimed to be advantageous when compared to any other colloidal carriers, and is known to combine the advantages and avoid the disadvantages associated with numerous colloidal carrier systems [3]. SLN does have several disadvantages e.g. unpredictable particle growth or unexpected drug expulsion from the lipid core are known to be common phenomena. Table 1 shows the advantages and disadvantages on the se of SLNs as drug delivery systems [4].
Table 1. Advantages and disadvantages of SLNs.
| Advantages | Disadvantages |
|
|
|
|
|
|
|
|
|
|
|
|
|
3. SLN preparation methods
3.1. High pressure homogenisation
High pressure homogeniser (HPH) proved to be a very effective dispersing technique in the preparation of SLNs. A reduction of the average particle size from 474 to 155 nm (for example) can be obtained after the first homogenisation cycle at 800 bars. The dispersing grade of SLN depends on the power density and the power distribution in the dispersion volume. High power densities result in more effective particle disruption. High pressure homogenisers reach by far the highest power densities (~1013 energy input W/m3). A homogeneous distribution of the power density is necessary to obtain narrow size distributions, otherwise, particles localised in different volumes of the sample will experience different dispersing forces and therefore the degree of particle disruption will vary within the sample volume. Inhomogeneous power distributions are observed in high-shear homogenisers and ultrasonifiers. HPHs are characterised by a homogenous power distribution due to the small size of the homogenising gap (25–30 mm) [3].
3.1.1. Hot homogenisation
High temperature is used in hot homogenisation techniques. The temperature is kept above the melting point of the lipid(s); drug is dissolved in the melted lipid(s) and the mixture is dispersed in a hot surfactant solution. The pre-emulsion is made by mixing drug, lipid(s) and surfactant, which is then prepared by using an ultrasonic pre-homogeniser for 3 min. The solution is then passed through a homogeniser for 5 min depending on the pressure [5].
3.1.2. Cold homogenisation
In the cold HPH technique, lipid is melted above its melting point and drug is dissolved or dispersed in it. The system is cooled down by means of dry ice or liquid nitrogen. After solidification, the lipid mass is grounded using ball or mortar milling to yield lipid micro-particles in a range between 50 and 100 µm. Then a micro-emulsion is formed by adding these micro-particles into cold surfactant solution with stirring. This suspension is passed through a HPH at/or below room temperature and the micro-particles are broken down to nanoparticles. Lipid particles prepared using the cold HPH technique possess a slightly higher polydispersity index (PI) and mean particle size compared to the ones obtained by hot HPH technique [6].
3.2. Solvent emulsification evaporation
In this method, the lipid is dissolved in water immiscible organic solvents (e.g. chloroform), which is then emulsified in an aqueous phase before evaporation of the solvent under condition of reduced pressure. This method is suitable for the incorporation of highly thermolabile drugs due to avoidance of heat during the preparation, however presence of solvent residues in the final dispersion may create problems due to regulatory concerns [6].
3.3. Micro-emulsion technique
A warm micro-emulsion is prepared containing molten lipid, surfactant and co-surfactant is added with stirring. This solution is then dispersed in cold water while stirring. Excess water can be removed by freeze drying. This method has certain advantages which include no need for specialised equipment, energy for the production is not required and scale-up production of lipid nanoparticles is possible. The main disadvantage of the microemulsion technique is the dilution of the particles suspension with water, thus removal of excess water need additional efforts. In addition, high concentrations of surfactants and co-surfactants, in the formulation raise regulatory concern [6].
3.4. Ultra-sonication
SLNs can be obtained by high speed stirring using ultra-sonication. This method was used for the production of an O/W emulsion in which high speed stirring was applied to the melted lipid phase and hot aqueous dispersion of surfactant. After cooling the resulting emulsion, solid particles of lipid were obtained. The main drawback of this method is the use of a high amount of surfactant without which the production of nanometre size particles is not possible. Physical instability and micro sized range of particles are some of the disadvantages of this technique [7].
3.5. Supercritical fluid method
This technique is considered to be a relatively new approach for SLN production. There are several variations in this platform technology for powder and nanoparticle preparation. In this method, a gas such as carbon dioxide is used as a solvent. SLN can be prepared by the rapid expansion of supercritical carbon dioxide solutions (RESS) method. The gas mainly used in this process is carbon dioxide because of its non-toxicity, low critical temperature and pressure. Some of the advantages of this technique include no usage of organic solvents and ability to produce nanoparticle and micro-particles in the form of dry powders [8].
3.6. Micro-emulsion technique
Warm water-in-oil-in-water (W/O/W) double micro-emulsions can be prepared in two steps. Firstly, w/o micro-emulsion is prepared by adding an aqueous solution containing drug to a mixture of melted lipid, surfactant and co-surfactant at a temperature slightly above the melting point of lipid to obtain a clear system. In second step, w/o prepared micro-emulsion is added to a mixture of water, surfactant and co-surfactant to obtain a clear w/o/w system. SLN can be obtained by dispersing the warm micro double emulsions in cold then washed with dispersion medium by ultra-filtration system. Multiple emulsions have inherent instabilities due to coalescence of the internal aqueous droplets within the oil phase, coalescence of the oil droplets, and rupture of the layer on the surface of the internal droplets. In case of SLN production, they have to be stable for few minutes, the time between the preparations of the clear double micro-emulsions and its quenching in cold aqueous medium, which is possible to achieve [8].
3.7. Solvent emulsification diffusion method
Using this method an average diameter of particle sizes between 50-100 nm can be obtained. One of the advantages of this technique is the avoidance of high temperatures during preparation. In this technique lipid is generally dissolved in the organic phase in a water bath at 50 °C and used as an acidic aqueous phase in order to adjust the zeta potential to form coacervation of SLN, and then separation can be done by centrifugation. The SLN suspension can be quickly produced. The entire dispersed system can then be centrifuged and re-suspended in distilled water [9].
4. Influence of ingredient composition on product quality
4.1. Ingredients
General ingredients for the production of SLN include solid lipid(s), emulsifiers and water. The term lipid is used here in a broad sense; these lipids can include triglycerides (e.g. tristearin), partial glycerides (e.g. Imwitor), fatty acids (e.g. stearic acid), steroids (e.g. cholesterol) and waxes (e.g. cetyl palmitate). Emulsifiers help to stabilise the lipid dispersion. It has been found that a combination of emulsifiers may prevent particle agglomeration [3]. Table 2 shows a list of excipients that can be used for the preparation of SLNs.
Table 2. An overview of ingredients that are commonly used for the preparation of SLNs.
| Triglycerides (lipids) | Hard fat types | Surfactants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
||
|
||
|
||
|
||
|
||
|
4.2. Influence of lipid
According to Muller et al., the critical parameters for the formation of nanoparticles are related to the usage of different lipids [10]. Some of the examples are the velocity of lipid crystallisation, the lipid hydrophilicity (influence on several self-emulsifying properties) and the shape of the lipid crystals, which in turn also influences the surface area. Moreover, most of the lipids used represent a mixture of several chemical compounds, and the quality of these chemical compounds can vary from different suppliers or vary for different batches from the same supplier. These small differences in the lipid composition can possibly have considerable impact on the quality of SLN dispersion (e.g. by changing the zeta potential, retarding crystallisation processes). According to Ahlin et al., lipid composition can supposedly influence the particle size of SLN that is produced by high shear homogenisation [11]. Increasing the lipid content over 5-10% in most cases results in larger particles (including micro-particles) and broader particle size distributions [12].
4.3. Influence of emulsifiers
The choice of the emulsifiers and their concentration is of great impact on the quality of the SLN dispersion [3]. Either surfactant or surfactant mixture affects the particle size of the lipid nanoparticles. According to Mehnert et al., SLN exhibited a much smaller particle size when the surfactant amount was increased [3]. The decrease in surfactant concentration resulted in increase of particle size during storage. One of the most characteristics of surfactant is that it decreases the surface tension between the interface of the particles causing portioning of the particles and thereby increasing the surface area [8].
5. Secondary production steps
5.1. Stability of the drug and SLNs
Stability considerations that are relevant to SLNs are the chemical stability of the drug and the physical stability of SLN. Prevention of degradation reactions such as hydrolysis is an important chemical stability parameter and examples for physical stability issues include the prevention of particle size growth and polymorphic changes of the solid lipid. Lipids and surfactants must be chosen carefully and should be mutually compatible to improve the chemical stability [13].
Particle size distribution determines the bio-distribution, shelf-life and route of administration of SLN formulation. The SLN dispersion should possess a narrow size distribution to avoid particle size growth due to Ostwald ripening. Ostwald ripening is a thermodynamically driven process, in which smaller particles dissolve and redeposit onto the surface of larger particles. This process occurs because smaller particles have larger surface area and higher surface energy and hence higher Gibbs free energy than the larger particles. All systems tend to attain lowest Gibbs free energy. In other words, larger particles are more energetically stable and favoured over smaller particles. Ostwald ripening can be reduced by minimising polydispersity in the particle size but it cannot be prevented [8].
In SLN dispersions there are three types of instabilities (creaming, flocculation and coalescence). In creaming process the less dense phase migrates to top of the dispersion under the influence of buoyancy or centripetal force. Creaming causes the SLNs to come close to each other which in turn also initiate Ostwald ripening, flocculation and coalescence. It has been reported that creaming phenomenon can be prevented by matching the density of the lipid and the aqueous phase. Flocculation is a process in which the nanoparticles are held together in loose associations by weak van der Waals forces. Coalescence is a process in which the nanoparticles fuse to form larger particles. The electrostatic repulsion and steric hindrance between particles produced in the presence of surfactants have been found to inhibit flocculation [14].
Electrostatic repulsion produces an electrical double layer around each nanoparticle in SLN dispersion. The electrical double layer comprises of two parts: an inner region (stern layer), in which the ions are tightly bound and an outer diffuse region, in which the ions are less firmly attached. A notional boundary forms between particles and ions within this diffuse layer. Ions within the boundary move with the particle and the ions outside the boundary do not move with the particle. This notional boundary is called as slipping plane. The potential at the slipping plane is known as zeta (ζ) potential. The magnitude of the ζ-potential is an important determinant of the stability of SLN dispersions. As the ζ-potential increases, the magnitude of electrostatic repulsion between the particles also increases, hence the particles will tend to repel each other and there is no tendency to flocculate. Colloidal dispersions with a ζ-potential of more positive than +30 mV and negative than -30mV are considered to be stable. Steric effects also play an important role in the stability of SLN dispersion by hindering the particles from coming closer to each other and thus preventing flocculation and coalescence. The polyoxyethylene chain present in non-ionic surfactants extends in the aqueous medium in the form of a coil and providing steric hindrance. Optimum surfactant concentration and sufficient chain length (≥ 20 ethylene oxide units) will impart steric effect mediated formulation stability. For long-term stability a balance between electrostatic repulsion and steric effect must be obtained.
5.2. Lyophilisation
Lyophilisation can play an important part to increase the chemical and physical stability of SLN over extended period of time. Lyophilisation had been required to achieve long term stability for a product containing hydrolysable drugs or a suitable product for per-oral administration. Transformation into the solid state would prevent the Oswald ripening and avoid hydrolytic reactions. In case of freeze drying of the product, all the lipid matrices used, can form larger solid lipid nanoparticles with a wider size distribution due to presence of aggregates between the nanoparticles. The conditions of the freeze-drying process and the removal of water promote the aggregation among SLNs. An adequate amount of cryo-protectant (such as sucrose, lactose, mannitol, polyethylene glycol etc.) can protect the aggregation of solid lipid nanoparticles during the freeze-drying process [15].
5.3. Spray drying
Compared to lyophilisation spray drying is cheaper. The usage of spray drying is more encouraged when the lipids melting point is more than 70 °C. According to Mehnert et al., the best results with spray drying were obtained with SLN concentration of 1% in a solution of trehalose in water or 20% trehalose in ethanol-water mixture [3]. The addition of carbohydrates and low lipid content favour the preservation of the colloidal particle size in spray drying. Freitas et al., illustrated that melting of the lipid can be minimised by using ethanol-water mixtures instead of pure water due to cooling leads to small and heterogeneous crystals [16].
6. Drug incorporation and release from SLNs
6.1. Drug incorporation
Three different models for the incorporation of active ingredients into SLN have been extensively studied [10]. These are homogeneous matrix model, drug-enriched shell model and drug-enriched core model (Figure 2). The structure that is obtained is a function of formulation composition, such as lipid, active compound, surfactant, and also of the production conditions (hot vs cold homogenisation). A homogeneous matrix with molecularly dispersed drug or drug being present in amorphous clusters is thought to be mainly obtained when applying the cold homogenisation method and when incorporating very lipophilic drugs in SLN with the hot homogenisation method [17]. During the cold homogenisation method, the bulk lipid matrix contains the dissolved drug in molecularly dispersed form, which afterwards is mechanically broken down high pressure homogenisation leading to the formation of nanoparticle. In hot homogenisation method the produced oil droplet is being cooled and crystallised. It is to note that no phase separation between lipid and drug occurs during the cooling process.
Drug enriched outer shell can be obtained when phase separation occurs during the cooling process from liquid oil droplet to the formation of SLNs. According to Muller et al., the lipid can precipitate first by forming a practically compound free lipid core [10]. Also during the forming process of lipid core, the concentration of active compound in the remaining liquid lipid increases continuously. Finally, the compound enriched shell crystallises, this model is assumed to give away a very fast release which is highly desired in various SLN applications. Core enriched with active compound can be formed when the opposite occurs, which means the active compound starts precipitating first and the shell will have distinctly less drug. This leads to a membrane controlled release governed by the Fick law of diffusion. The structure of SLN formed clearly depends on the chemical nature of active compound and excipients and the interaction thereof. In addition, the structure can be influenced or determined by the production conditions.
There are two main methods drug loading, passive loading and active or remote loading. Passive loading involves the loading of the entrapped agents before or during the manufacturing process. The alternative method, active or remote loading enables the entrapped agents to be introduced into the lipid formulations after the intact vesicles are formed. This method allows compounds with ionisable groups and amphiphilic molecules to be introduced. The active / remote loading method offers many advantages over the passive method, most importantly its high encapsulation efficiency. It produces a lipid particle with reduced leakage of the encapsulated compound and it avoids the handling of biologically active compounds during the preparation process.
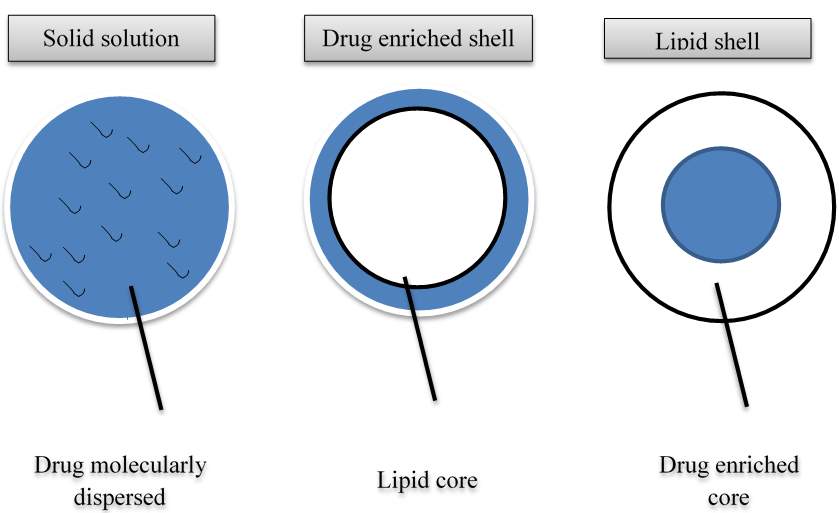
Figure 2. Illustration of different drug incorporation modes.
6.2. Drug release from SLNs
Üner et al., have intensively investigated the effect of formulation parameters and production conditions on the release profile of SLNs [18]. For example, they investigated the release profile as a function of production temperature. In most cases, burst release is observed from SLNs. An initial burst release is afterwards followed by a prolonged release, this process is called biphasic. It has also been observed that the burst release phenomenon only takes place when hot homogenisation is used and very high temperatures are applied. For particles that travel through circulation system, prolonged release is desired [19]. Fabricated Doxorubicin SLNs with mean diameter of 199 nm using glyceryl caprate and dimethyl sulfoxide via high pressure homogenisation method, by prolonged the release of doxorubicin was observed by Xie et al. [20]. Doxorubicin-loaded SLNs were fabricated using tetradecanoic acid, palmitic acid, stearic acid respectively. On the other hand, biphasic release patterns are completely non-existent when cold homogenisation is used. The release kinetics can also depend on the release conditions (e.g. sink or non-sink conditions, release medium).
Surfactant concentration can also play a vital role in burst release, and different studies have suggested that high surfactant concentration can lead to high burst release. This was explained by redistribution effects of the active compound between the lipid and the water phase during the heating up process and subsequently the cooling down process after production of the hot o/w emulsion during the hot homogenisation process. Solubility of active compound in the water phase increases due to the heating of lipid/water mixture and the compound partitions from the melted lipid droplet to the water phase. After the homogenisation procedure is done the lipid core starts to crystallise with still a relatively high number of active compounds in the water phase. Further cooling leads to super saturation of the compound in the water phase, then the compounds partitions back into the lipid phase; a solid core has already started forming leaving only the liquid outer shell for compound accumulation. Therefore, it can be observed that as the solubility in water phase goes higher so does the burst effect, and the solubility increases drastically when increased temperature and increased surfactant concentrations are used [3].
7. Biological and pharmaceutical aspects of SLNs
Advances in biocompatible nanoscale drug carriers such as liposomes and polymeric nanoparticles, have enabled more efficient and safer delivery of numerous of drugs. Lipid drug delivery systems currently serve as a useful tool for the delivery of active anticancer agents and have been used successfully used as drug carriers in the treatment of many cancers, where many clinical studies have shown enhanced therapeutic activity of the drug compared with the free drug. Moreover, lipid based drug delivery systems are able to modify the pharmacokinetics and pharmacodynamics of cancer cells, allowing them to increase the localised concentration of the drug released into the target cell without losing their properties and reduce the exposure of normal cells to the drug molecules. Lipids can also be served as a means to deliver actives by both active and passive targeting to cancer cells and targeting of cancer cells using the Enhanced Permeability and Retention (EPR) effect. As with most anticancer agents, there is a need to attain a local and targeted delivery of the active agent and one of the unique properties of lipids are their natural ability to target cancer cells due to their enhanced permeability across cell membranes.
SLN is considered to be suitable also for parenteral drug delivery because of their small size. SLN may be injected intravenously (IV) and used to target drugs to particular organs. Upon administration into the systemic circulation, several colloidal carriers such as liposomes and polymeric nanoparticles are rapidly cleared by cells of reticuloendothelial system (RES). RES usually resides in the spleen, liver and in the form of Kupffer cells in the liver consisting of phagocytic cells, which is considered to be the major part of the immune system. RES is known to remove drug carriers within minutes identified as foreign objects [21].
Clearance by RES is beneficial only when the spleen or liver, lymph nodes are the target tumour site for other type of cancers RES is known to be the major barrier. When colloidal carrier surface is modified with hydrophilic polymer it increases the blood circulation time and resistance to clearance by RES. This type of surface modification of drug delivery systems by polymers is called long circulating drug carriers. Polyethylene glycols (PEG) have been employed widely to get the stearic stabilisation of colloidal carriers. PEGs are electrical neutrality, chain flexibility, lack of functional group and high hydrophilicity which prevent it from interacting with biological components unnecessarily. Other hydrophilic molecules have been tried are Brij 68, Brij 78, and Pluronic F188 [22]. In order to facilitate drug targeting a reticuloendothelial system avoidance facility these block polyoxyethylene (POE) polypropylene copolymers like Pluronic F188 can be used, in which the hydrophobic portion of the molecule forms the nanoparticle matrix while the water-soluble POE block forms a hydrophilic coating on the SLN increase the tumour accumulation and anticancer activity of drugs [23].
The administered particles are cleared from the circulation by liver and spleen because of the small size of SLN (below 1 μm), these lipid nanoparticle formulations can be used for systemic body distribution with a minimal risk of blood clotting and aggregation, which can lead to embolism. SLN can also provide a sustained release of drug when administered IV. Drug encapsulated inside the lipid core can be released gradually on erosion (e.g. degradation by enzymes) or by diffusion from the particles [24].
8. Use of SLN in various cancer therapies
Cancer is a condition where cells in a specific part of the body both grows and reproduces in an uncontrollable manner. The cancerous cells are known to invade and destroy surrounding healthy tissues, which includes organs as well. Apart from a few cancer types (e.g. breast cancer), for which hormonal therapy or immunotherapy is used, cytotoxic drugs are used as the major form of chemotherapy for cancer [25]. Particulate drug carrier systems can have a great promise to improve the therapeutic effectiveness and safety profile of this conventional form of cancer chemotherapy [26]. Because of the numerous advantages that SLN can offer, this relatively new drug carrier is considered be an emerging drug carrier system in the field of anticancer drug delivery.
A tumour is normally associated with a defective, leaky vascular architecture as a result of the poorly regulated nature of tumour angiogenesis. Moreover, the interstitial fluid within a tumour is usually inadequately drained by a poorly formed lymphatic system. Due to this phenomenon, submicron sized particulate matter may preferentially extravasate into the tumour and be retained. This is often quoted as the “enhanced permeability and retention” (EPR) effect. This EPR effect can be taken advantage of by a properly designed nanoparticle system such as SLN to achieve passive tumour targeting. By doing this, the aforementioned poor tissue specificity problem can be partly solved. Furthermore, with the advances in surface-engineering technology, the biodistribution of SLN can be further manipulated by modifying the surface Physicochemical properties of SLN to target them to the tissue of particular interest [3]. As a result of this, the chances of drugs reaching the tumour sites can be further enhanced and systemic drug toxicity can be reduced. Cytotoxic drug delivery systems such as polymeric nanoparticles and liposomes possess several problems in terms of physical stability, protection of labile drugs from degradation and controlled release. SLN tends not to demonstrate such disadvantages [24]. Delivery of drugs from active form to solid tumours can be difficult. It is known that most anticancer agents have a large volume if distribution upon administration and subsequently narrow therapeutic index due to a high level of toxicity in healthy tissues. Through the successful encapsulation of these drugs in a drug delivery system, such as SLN, the volume of distribution can be significantly reduced and the concentration of drug in the tumour site can be increased [27]. SLN formulations of different anticancer agents have been shown to be less toxic than the free drug. Table 3 shows a list of anti-cancer drug encapsulated SLNs. Although there is still a lack of clinical studies of the use of SLN for cancer treatment, preclinical studies using cell culture systems or animal models have so far been very promising [28].
Table 3. A summary of SLN formulations used for the delivery of drugs with anticancer properties and the significant works based on these formulations (adapted from [26]).
8.1. Brain tumour
The incidences of primary brain tumours in the United States have been estimated at approximately 43,800 per year. SLN is considered to be one of the best nanoscale lipid based compound for brain tumour drug delivery. Although the exact mechanism by which these SLNs cross Blood Brain Barrier (BBB) is still unknown, internalisation is hypothesised to be mediated by endocytosis of SLNs by endothelial cells. Endocytosis procedure is initiated by the successful absorption of circulating plasma proteins to the SLN surface [29]. Drug loading and any sort of possible drug degradation can be altered by lipid matrix. Drug unloading within the tumour tissues can also be controlled. This control can be achieved by the respective surface coating of the SLN with these constituent lipids [24]. Different studies suggested that nanoparticles with tween surfactants, resulted in transport of drugs across the BBB [30]. SLN have the potential to revolutionise both preoperative and intraoperative brain tumour detection.
Moreover, delivery of drugs to brain is a subtle task in the therapy of many severe neurological disorders, with SLNs easily diffuse the BBB due to their lipophilic nature. According to Singh et al. ligand conjugation on SLN surface enhances the targeting efficiency, by reporting Lactoferin (Lf) conjugated SLN system for effective brain targeting [31]. A study by Neves et al., has used resveratrol-loaded SLNs that have been functionalised with apolipoprotein E, and show that these systems might be a promising strategy for resveratrol delivery into the brain [32]. Graverini et al., have reported the preparation of SLNs to deliver andrographolide (AG) into the brain [33]. SLNs were prepared using Compritol 888 ATO as solid lipid and Brij 78 as surfactant and applying emulsion/evaporation/solidifying method as preparative procedure. The ability of SLN to cross the BBB was evaluated in vitro and in vivo in healthy rats.
8.2. Breast cancer
Breast cancer is the most common malignancy in women in most countries of the world and is one of the cancers most frequently observed in industrialised countries. There are over 1.4 million cases of cancer worldwide, 30% of which involve breast cancer with approximately 50,000 and 400 new cases in women and men respectively, diagnosed with the disease each year in the UK (Cancer Research UK). In recent decades, the death rate in breast cancer patients has been significantly reduced due to the implementation of periodic check-ups and treatments such as chemotherapy, hormone therapy, radiotherapy, and surgery.
Among the major challenges in effective breast cancer chemotherapies are inadequate drug concentrations reaching the tumour, their rapid elimination, systemic toxicity and adverse effects SLN has the potential to overcome current chemotherapeutic barriers in breast cancer treatment and to solve the problems associated with traditional chemotherapy and multidrug resistance [34]. Chemo-resistance or multidrug resistance can generally result from either of the two means firstly by physically impairing delivery to the tumour (e.g. poor absorption, increased metabolism/excretion, and/or poor diffusion of drugs into the tumour mass), and secondly through intracellular mechanisms that raise the threshold for cell death. Nanoparticles are beneficial tumour targeting vehicles due to their passive targeting properties by the EPR effect.
Campos et al., investigated the potential of modified SLNs for the delivery of paclitaxel (PAX) and found that these systems were stable and reproducible [35]. Their funding’s have reveal that chitosan-hyaluronic-coated SLNs facilitated the targeting, cellular uptake and the time-/dose-controlled delivery and release of PAX. Wang at al., have formulated resveratrol-loaded SLNs to treat MDA-MB-231 cells, and by comparing these with free resveratrol has shown that the Res-SLNs displayed a superior ability in inhibiting the proliferation of MDA-MB-231 cells [36]. Another added advantage in SLN drug delivery system is the development of proteins (transferrin) and commercial antibody conjugated SLN formulations for active targeting of breast cancer [37].
8.3. Colorectal cancer
Colorectal cancer (CRC) ranked second in females and third in males among all type of cancers diagnosed in United States, and also known to be the most common cancer in several other western countries. SLN have been proposed as a new approach of drug carrier for colorectal cancer. SLN carrying cholesteryl butyrate, doxorubicin and paclitaxel had previously been developed [38]. Rajpoot & Jain developed and optimized oxaliplatin (OP) loaded SLNs containing tristearin, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), Lipoid S75, and Tween 80 [39]. They have shown that drug entrapped in OP-SLNs have higher sensitivity of HT-29 cells compared to OP-SLNs and OP solution, which is a promising approach for the management of CRC. Other studies have shown that 5-fluorouracil (5-FU) loaded SLNs can produce superior anticancer activity than pure 5-FU for the treatment of CRC [40].
8.4. Lung cancer
One of the leading causes of cancer related deaths worldwide is lung cancer. Adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma, which together make up the majority of lung cancers, are referred to as ‘‘non-small cell lung cancers’’ (NSCLCs). Patients with early stage NSCLC are typically treated with surgery with a 5-year survival rate ranging from 25% to 80%. This survival rate also depends vastly on the stage of the disease. The causes of lung cancers are generally characterised by mutations in p53 gene. These mutations can lead to an exponential loss of tumour-suppressor function, increase drug resistance, loss of mutational repair, increase of angiogenesis, inhibition of cells and proliferation of apoptosis. SLNs have gained increasing attention as a promising colloidal carrier system. As a substitution for viral delivery systems it was reported the use of p53 gene/cationic lipid complexes for the treatment of early endobronchial cancer [41]. Bakhtiary et al., reported the development of erlotinib (ETB) loaded SLNs based formulation of dry powder inhaler (DPI), which displayed a suitable flowability and aerodynamic traits and by Next Generation Impactor (NGI) analyses have confirmed deep inhalation pattern of the formulations [42].
8.5. Prostate cancer
Prostate cancer is known to be one of the most important cancer in men, especially in the industrialised countries of the western world. According to several reports the incidence of prostate cancer has been increasing over the last decade. The reason behind this rapid increase of incidence is thought to be due to the heterogeneous and peculiar nature of individual cancers, and moreover the inability to target therapies to neoplastic cells [43]. The usage of SLN as a drug delivery system considered to be advantageous for inhibition of prostate cancer cells, such as LNCaP. One of the many advantages of using SLN is the usage of well tolerated compound’s while making these nanoparticles, and moreover the avoidance of organic solvents, alongside good physical stability. SLN have a great potential as a drug delivery system for prostate cancer cells since it is capable of enhanced accumulation in the target tissue through a passive targeting mechanism (e.g. enhanced permeability and retention effect, EPR), an increased accumulation and cell uptake through receptor-mediated endocytosis can be obtained if specific binding agents are attached on the surface of SLNs.
Akanda et al., reported the formulation of retinoic acid (RTA) loaded SLNs by tuning the process parameters (e.g. pressure/temperature) and by using different lipids to develop nanodispersions with enhanced anticancer activity [44]. The RTA-SLN dispersions were produced by high-pressure homogenisation and the anticancer efficiency was evaluated by incubating RTA-SLNs with human prostate cancer (LNCap) cells, which demonstrated reduced cell viability with increased drug concentrations (e.g. 9.53% at 200 μg/ml) while blank SLNs displayed negligible cytotoxicity.
9. Marketed products and current studies on SLN
Since early nineties, researchers turned their attention to lipid nanoparticles because of their nontoxicity and cost/effectiveness relationship. In spite of the advantages, formulating with lipid nanoparticles has been suffering some drawbacks. Because of the gastro intestinal tract (GIT) conditions, most of promising drugs do not reach clinical trials. The stability of particles must be comprehensively tested due to pH changes and ionic strength as well as the drug release upon enzymatic degradation [45]. Lipid nanoparticles absorption through GIT occurs via transcellular (through M cells or enterocytes) or paracellular (diffusion between cells). If the major drug uptake occurs through M cells, the portal vein to the liver is bypassed, resulting in higher drug concentrations to the lymph rather than to plasma [46].
Despite the low number of lipid nanoparticles formulations on the market for drug delivery, Mucosolvan retard capsules (Boehringer Ingelheim) is a story of success. Mucosolvan retard capsules was the first generation. It was produced by highspeed stirring of a melted lipid phase in a hot surfactant solution obtaining an emulsion. This emulsion was then cooled down to room temperature obtaining the so-called “lipid nanopellets for oral administration”. Successful in vivo studies also include rifampicin, isoniasid, and pyrazinamide that are used in tuberculosis treatment. These drugs achieved higher bioavailability when incorporated into SLN compared to the free solutions. Rifampicin has poor cellular penetration which requires high doses to reach effective concentrations. Rifamsolin is a rifampicin-loaded SLN under preclinical phase by AlphaRx. The methodology employed for production is acceptable by the regulatory agencies and has been addressed by various papers and patents [47]. Poor water-soluble drugs, such as camptothecin, vinpocetine, and fenofibrate, can have their solubilisation improved if incorporated into SLN. Potta et al., in two studies in 2012 have developed SLNs for enhanced the solubility of poorly soluble drugs such as Cyclosporine (CyA) [48] and ibuprofen [49].
Another example is insulin, commonly administered parenterally in the treatment of diabetes mellitus. Injections are often painful and must be administered daily, which result in low patient compliance. Unfortunately, oral administration of insulin, produced by solvent emulsification-evaporation method based on a w/o/w double emulsion, has limitations such as low bioavailability due to degradation in the stomach, inactivation and degradation by proteolytic enzymes, and low permeability across the intestinal epithelium because of lack of lipophilicity and high molecular weight [46]. The main advantages of incorporate insulin into SLN would be the enhancement of trans mucosal transport and protection from the degradation in the GIT. New investigations will need to be designed with the aim of developing anti-cancer drug encapsulated SLNs which are capable of enhancing the bioavailability of the drug molecule.
10. Conclusions
The fast-developing science of nanotechnology is one of many areas that are expected to have a significant impact on medicine and how medicine delivered. There are several varieties of particles available, however, SLNs have attracted special interest in the last few decades and can be produced by different methods with many advantages, and have a great potential to be used as vehicle for the treatment of cancer with least side effects.
References
- Pardeike, J., Hommoss, A., Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. International journal of pharmaceutics 2009, 366(1), 170-184.
- Schubert, O., Wretlind, A. Intravenous infusion of fat emulsions, phosphatides and emulsifying agents. Acta Chir Scand Suppl 1961, 13, 278.
- Mehnert, W., Mäder, K. Solid lipid nanoparticles: production, characterization and applications. Advanced drug delivery reviews 2001, 47(2), 165-196.
- Mukherjee, S., Ray, S., Thakur R.S. Solid lipid nanoparticles: Modern formulation in drug delivery system. Indian journal of Pharmaceutical Sciences 2009, 71(4), 349-358.
- Almeida, A.J., Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Advanced drug delivery reviews 2007, 59(6), 478-490.
- Chaturvedi, S.P., Mishra, A Production technique of lipid nanoparticles. Research Journal of pharmaceutical, biological and chemical sciences 2012, 3(3), 525-541.
- Souto, E. B., Muller, R.H. Lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers) for cosmetic, dermal, and transdermal applications. Drugs and the pharmaceutical sciences 2007, 166, 213.
- Ekambaram, P., Sathali, A.A.H, Priyanka, K. Solid lipid nanoparticles: A review. Scientific reviews and chemical communications 2011, 2(1), 80-102.
- Trotta, M., Debernardi, F., Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. International journal of pharmaceutics 2003, 257(1), 153-160.
- Muller, R.H., Mader, K., Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. European journal of pharmaceutics and biopharmaceutics 2000, 50(1), 161-177.
- Ahlin, P., Kristl, J., Smid-Korbar, J. Optimization of procedure parameters and physical stability of solid lipid nanoparticles in dispersions. Acta pharmaceutica 1998, 48(4), 259-267.
- Siekmann, B., Westesen, K. Melt-homogenized solid lipid nanoparticles stabilized by the nonionic surfactant tyloxapol. I. Preparation and particle size determination. Pharmacopeia pharmacological letters 1994, 1(3), 123-12694-197.
- Lim, S.J., Lee, M.K., Kim, C.K. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. Journal of controlled release 2004, 100(1), 53-61.
- Porter, M.R. Handbook of surfactants. 1994.
- Ohshima, H., Miyagishima, A., Kurita, T., Makino, Y., Iwao, Y., Sonobe, T., Itai, S. Freeze-dried nifedipine-lipid nanoparticles with long-term nano-dispersion stability after reconstitution. International journal of pharmaceutics 2009, 377(1), 180-184.
- Freitas, C., Muller, R.H. Spray-drying of solid lipid nanoparticles (SLN TM). European Journal of Pharmaceutics and Biopharmaceutics 1998, 46(2), 145-151.
- Yadav, N.E.H.A., Khatak, S.U.N.I.L., Sara, U.V. Solid lipid nanoparticles: a review. International Journal of pharmaceutics 2013, 5(2), 8-18.
- Üner, M., Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. International journal of nanomedicine 2007, 2(3), 289.
- Subedi, R.K., Kang, K.W., Choi, H.K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. European journal of pharmaceutical sciences 2009, 37(3), 508-513.
- Xie, S., Zhu, L., Dong, Z., Wang, X., Wang, Y., Li, X., Zhou, W. Preparation, characterization and pharmacokinetics of enrofloxacin-loaded solid lipid nanoparticles: influences of fatty acids. Colloids and surfaces biointerfaces 2011, 83(2), 382-387.
- Moghimi, S.M., Szebeni, J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in lipid research 2003, 42(6), 463-478.
- Chimmiri, P., Rajalakshmi, R., Mahitha, B., Ramesh, G., Noor Ahmed, V.H. Solid lipid nanoparticles: a novel carrier for cancer therapy. International Journal of biological pharmaceutical research 2012, 3(3), 405-413.
- Zara, G.P., Cavalli, R., Fundaro, A., Bargoni, A., Caputo, O., Gasco, M.R. Pharmacokinetics of doxorubicin incorporated in solid lipid nanospheres (SLN). Pharmacological research 1999, 40(3), 281-286.
- Wissing, S.A., Kayser, O., Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Advanced drug delivery reviews 2004, 56(9), 1257-1272.
- Ewesuedo, R.B., Ratain, M.J. Principles of cancer chemotherapy. In Oncologic Therapies Publisher: Springer Berlin Heidelberg, 2003, pp. 19-66.
- Wong, H.L., Bendayan, R., Rauth, A.M., Li, Y., Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Advanced drug delivery reviews 2007, 59(6), 491-504.
- Speth, P.A.J., Van Hoesel, Q.G.C.M., Haanen, C. Clinical pharmacokinetics of doxorubicin. Clinical pharmacokinetics 1998, 15(1), 15-31.
- Pardeshi, C., Rajput, P., Belgamwar, V., Tekade, A., Patil, G., Chaudhary, K., Sonje, A. Solid lipid based nanocarriers: An overview/Nanonosači na bazi čvrstih lipida: Pregled. Acta pharmaceutica 2012, 62(4), 433-472.
- Nagayama, S., Ogawara, K.I., Fukuoka, Y., Higaki, K., Kimura, T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. International journal of pharmaceutics 2007, 342(1), 215-221.
- Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. Journal of nanoscience and nanotechnology 2004, 4(5), 484-488.
- Singh, I., Swami, R., Pooja, D., Jeengar, M.K., Khan, W., Sistla, R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. Journal of Drug Targeting 2016, 24(3).
- Neves, A.R., Queiroz, J.F., Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. Journal of Nanobiotechnology, 2016, 14, 27. doi:10.1186/s12951-016-0177-x.
- Graverini, G., Piazzini, V., Landucci, E., Pantano, D., Nardiello, P., Casamenti, F., Pellegrini-Giampietro, D.E., Bilia, A.R., Bergonzi, M.C. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: in vitro and in vivo evaluation. Colloids Surf B Biointerfaces. 2017, 27(161), 302-313. doi: 10.1016/j.colsurfb.2017.10.062.
- Bassiouni, Y., Faddah, L. Nanocarrier-Based Drugs: The Future Promise for Treatment of Breast Cancer. Journal of applied pharmaceutical science 2012, 2(05), 225-232.
- Campos, J., Varas-Godoy, M., Haidar, Z.S. Physicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: a proof-of-concept study in breast cancer cells. Nanomedicine 2017, 12(5), 473-490.
- Wang, W., Zhang, L., Chen, T., Guo, W., Bao, X., Wang, D., Ren, B., Wang, H., Li, Y, Wang, Y., Chen, S., Tang, B., Yang, Q., Chen, C. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814.
- Du, W., Hong, L., Yao, T., Yang, X., He, Q., Yang, B., Hu, Y. Synthesis and evaluation of water-soluble docetaxel prodrugs-docetaxel esters of malic acid. Bioorganic & medicinal chemistry 2007, 15(18), 6323-6330.
- Nielsen, D., Maare, C., Skovsgaard, T. Cellular resistance to anthracyclines. General Pharmacology: The vascular system 1996, 27(2), 251-255.
- Rajpoot, K., Jain, S.K. Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: preparation, optimization, and in vitro evaluation. Artificial Cells, Nanomedicine, and Biotechnology 2017, In Press. doi: 10.1080/21691401.2017.1366338.
- Patel, M.N., Lakkadwala, S., Majrad, M.S., Injeti ER, Gollmer, S.M., Shah, Z.A., Boddu, S.H., Nesamony, J. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS PharmSciTech 2014, 15, 1498-1508.
- Zou, Y., Zong, G., Ling, Y.H., Hao, M.M., Lozano, G., Hong, W.K., Perez-Soler, R. Effective treatment of early endobronchial cancer with regional administration of liposome-p53 complexes. Journal of the national cancer institute 1998, 90(15), 1130-1137.
- Bakhtiary, Z., Barar J., Aghanejad, A., Saei, A.A., Nemati, E., Ezzati Nazhad Dolatabadi, J., Omidi, Y. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Development and Industrial Pharmacy 2017, 43(8), 1244-1253.
- Sanna, V., Lubinu, G., Madau, P., Pala, N., Nurra, S., Mariani, A., Sechi, M. Polymeric Nanoparticles Encapsulating White Tea Extract for Nutraceutical Application. Journal of agricultural and food chemistry 2015, 63(7), 2026-2032.
- Akanda, M.H., Rai, R., Slipper, I.J., Chowdhry, B.Z., Lamprou, D.A., Getti, G., Douroumis, D. Delivery of retinoic acid to LNCap human prostate cancer cells using solid lipid nanoparticles. Int. J. Pharm. 2015, 493 (1-2), 161-171.
- Mathur, V., Satrawala, Y., Rajput, M.S., Kumar, P., Shrivastava, P., Vishvkarma, A. Solid lipid nanoparticles in cancer therapy. International journal of drug delivery 2011, 2(3), 212-223.
- Harms, M., Müller-Goymann, C.C. Solid lipid nanoparticles for drug delivery. Journal of drug delivery science and technology 2011, 21(1), 89-99.
- Wei, W., Shi, S. J., Liu, J., Sun, X., Ren, K., Zhao, D., …Gong, T. Lipid nanoparticles loaded with 10-hydroxycamptothecin–phospholipid complex developed for the treatment of hepatoma in clinical application. Journal of drug targeting 2010, 18(7), 557-566.
- Potta, S.G., Minemi, S., Nukala, R.K., Peinado, C., Lamprou, D.A., Urquhart, A.J., Douroumis, D. Development of solid lipid nanoparticles for enhanced solubility of poorly soluble drugs. J. Biomed. Nanotech. 2010, 6(6), 634-640.
- Potta, S.G., Minemi, S., Nukala, R.K., Peinado, C., Lamprou, D.A., Urquhart, A.J., Douroumis, D. Preparation and characterization of Ibuprofen solid lipid nanoparticles with enhanced solubility. J. of Microencapsulation 2010, 28(1), 74-81.
© 2017 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




