Amyotrophic Lateral Sclerosis Epidemiology, Etiology, Pathological Mechanism, Diagnosis and Treatment
Info: 11376 words (46 pages) Dissertation
Published: 10th Dec 2019
Tagged: Medicine
- INTRODUCTION
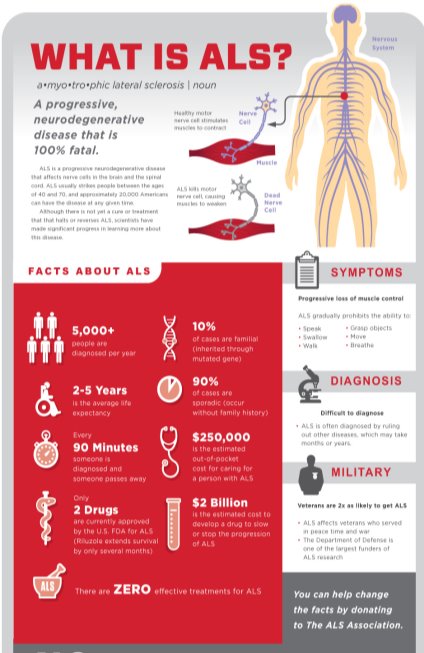
Amyotrophic lateral sclerosis or commonly known as ALS is one of the major neurodegenerative diseases alongside Alzheimer’s disease and Parkinson’s disease in the United States. A-myo-trophic is derived from the Greek language. A meaning no, Myo meaning muscle, and Trophic meaning nourishment that translates to No-muscle-nourishment. With no nourishment, the tissues degenerate leading to scarring or sclerosis of the region. Lateral indicates the location in the spinal cord, responsible for effective functioning of neurons. [1] ALS is a progressive disorder that involves degeneration of the upper motor neurons (UMN) in the frontal lobe of the brain and the lower motor neurons (LMN) in the brain stem and the spinal cord.
In ALS, as motor neurons die, a person loses the ability to walk, speak, swallow, and breathe. As the degeneration advances, the muscles gradually weaken and atrophies, losing its ability to control voluntary movements. ALS may rarely impair a person’s mind or personality, but people with ALS develop cognitive problems involving memory, speech fluency, and decision-making. ALS is usually fatal within 2-5 years of diagnosis. [2]
- BACKGROUND
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal neuromuscular disease. Jean-Martin Charcot first described the disease in 1869. He used ALS as a prototypic example of his research techniques to coin the term “méthode anatomoclinique.” This method provided a disciplined and systematic approach to classify neurological diseases based on integrating clinical signs and anatomical lesions. [3] Because of Charcot’s fundamental contributions, the term “Charcot’s disease” is used as a synonym for amyotrophic lateral sclerosis.
ALS is also referred to as ‘Lou Gehrig’s Disease’ as it caused the death of the New York Yankees baseball player, Lou Gehrig in 1939. ALS can affect any human being regardless of their age, sex, or ethnic groups. For example, the famous astrophysicist Stephen Hawking was diagnosed with ALS at a young age and has survived for over 50 years. Mao Zedong, founder of the People’s Republic of China, Lane Smith- an American actor, O.J. Brigance- a professional football player, have been victims of ALS. [1]
In the summer of 2014, social media was taken by storm with videos of people pouring ice water on themselves for the Ice Bucket Challenge. This initiative was introduced by Pete’s Frates, a professional baseball player, who helped in increasing awareness for ALS and raised millions of dollars for research. The Ice Bucket Challenge was an enormously successful Internet phenomenon accepted by many actors, philanthropist, artists, and athletes. [4] Over 17 million people uploaded their challenge videos to various social media sites, and these videos were watched by 440 million people! The ALS Association collected $115 million in a six-week span from the ALS Ice Bucket Challenge. The ALSA reports that a majority of this fund was allocated for research and the rest was used for patient and community services, education, and fundraising. [4], [5]
- EPIDEMIOLOGY
ALS is the most frequent adult-onset motor neuron disease. It is characterized by both upper and lower motor neuron degeneration and has a median survival of 2–5 years. [2] The worldwide annual incidence of ALS is about 1.9 per 100,000 individuals. Since, almost all patients with ALS die of their disease, mortality rates for ALS individuals remains constant. As per the recent analysis, the number of ALS cases worldwide is predicted to rise by 69% over the next 25 years. According to the United Nations, the number of individuals above age 60 is expected to increase rapidly. This increase is particularly due to improving healthcare and economic conditions among developing nations. [6]
- Age
ALS can strike at any age, although symptoms develop as one grows older. Mean age at onset is 58 to 63 years for sporadic ALS and 40 to 60 years for familial ALS. [2] As per the Centre for Disease Control and Prevention results, individuals with those aged 18 to 39 years had the least prevalence rate (0.5 per 100,000 persons), and the age group 70 to 79 years had the highest prevalence rate (17.0 per 100,000 persons). [7], [8] The prevalence of ALS increases with age.
- Gender
It is observed that men are at a higher risk to develop ALS than women, leading to a male-to-female ratio of 1.2–1.5. [7] Although as age increases, the incidence of ALS between men and women disappears. Few studies suggest that military veterans are twice more prone to ALS, especially those deployed during the Gulf War. [2] Possible risk factors for veterans include exposure to lead, pesticides, and diverse environmental toxins.
- Race and ethnicity
ALS can affect any person with no racial, ethnic, or socioeconomic boundaries. The prevalence rate for Caucasians was 2-fold greater than in African-Americans. Caucasians have a prevalence rate of 4.2 per 100,000 as compared to 2.0 per 100,000 for African-Americans. [7] Some geographic regions have an unusually high incidence of ALS, specifically Guam and the Kii Peninsula in Japan. The incidence of ALS in these regions is high due to environmental factors, especially a neurotoxic non-protein amino acid, β–methylamino-L-alanine (BMAA) produced in the seeds of Cycas micronesica. [2] It is hypothesized that patients in these regions may have a genetic susceptibility because of their inability to prevent BMAA accumulation.
- CLINICAL FEATURES & SYMPTOMS
Amyotrophic lateral sclerosis is a condition involving both UMN and LMN. The progression and spread of the disease can be both local and between neuro-anatomically linked regions. The identification of specific phenotypes helps in prognosis and survival, and also for their enrolment in clinical trials. The clinical manifestation of ALS helps in learning about the progression of the disease in an affected individual. The important presentations of ALS are described below;
- Limb onset ALS
Limb-onset ALS is the dominant feature with 70% of the cases among patients. [9] The main clinical feature in Limb-onset ALS is a combination of UMN and LMN damage involving brainstem and spinal cord regions. Patients with lower limb onset may complain of tripping, stumbling while walking or running. Patients with upper limb onset face difficulty in performing actions such as eating, writing, or picking up small objects.
- Bulbar onset ALS
Patients with bulbar onset present both upper and lower motor neuron signs. Dysarthria is a characteristic feature in patients with bulbar-onset ALS. Bulbar upper motor neuron symptoms include speech problems such as slurring, hoarseness, drooling, and distorted speech are typical symptoms. [10] Bulbar lower motor neuron signs include tongue wasting, fasciculations, and flaccid dysarthria. Some ALS patients show pseudo-bulbar symptoms like exaggerated involuntary emotional responses. Episodes of intense laughter may be followed at once by tears.
- Primary lateral sclerosis
Primary lateral sclerosis is a variant of ALS with UMN involvement. It is a slow progressive type but affects the whole body. It is a rare motor neuron disease and spreads from the bulbar region to limbs. [9], [10] Primary lateral sclerosis is characterized by spasticity, weakness, pathologically hyperreflexia, and pseudo-bulbar speech.
- Progressive muscular atrophy
Progressive muscular atrophy is another variant of ALS, represented by progressive LMN signs without clinical evidence of UMN dysfunction. [9] Symptoms of this disease are fasciculation, atrophy, and muscle weakness.
- ETIOLOGY
Although the etiology of ALS is not entirely understood, it can be classified into Familial ALS and Sporadic ALS. This section explains in brief the genetic and non-genetic causative agents for ALS.
5.1 Familial ALS (FALS)
Familial ALS means that there is more than one occurrence of the disease in a family. FALS represents about 5 ~ 10% of all ALS cases diagnosed. [11] FALS can be further categorized by mode of inheritance and sub-categorized by the specific gene. Over 25 causative genes have been linked to hereditary ALS. FALS can be inherited in an autosomal dominant, autosomal recessive, or X-linked manner depending on the gene involved.
5.1.1 Autosomal Dominant (AD)
Autosomal dominance is a characteristic pattern of inheritance wherein a single copy of the disease-associated gene is sufficient to cause the disease. A few examples of AD genes are Superoxide dismutase1 (SOD1), Fused in sarcoma (FUS), TAR DNA-binding protein (TARDBP), and Chromosome 9 open reading frame 72 (C9orF72).
- ALS1: Superoxide dismutase 1 (SOD1): SOD1 gene can be described as an autosomal dominant and autosomal recessive in the FALS pedigree. Mutations in SOD1 gene accounts for 20% of familial ALS and 5% of sporadic disease. [12] SOD1 converts harmful superoxide radicals to molecular oxygen and hydrogen peroxide thus protecting cells from the accumulation of free radicals. [13] Mutations in SOD1 impair the effective functioning of this protein.
- ALS6: Fused in Sarcoma (FUS): FUS is a nucleoprotein that is responsible for DNA repair, regulation of transcription, and RNA splicing. Over 60 FUS mutations have been identified in 3-5% of patients with familial ALS and in 1% of patients with sporadic ALS. [12] ALS-linked mutations in the FUS gene disrupt the effective functioning of DNA and RNA metabolism in the cell. The phenotypes associated with FUS mutations include adult-onset ALS, Juvenile-ALS, ALS-Frontotemporal dementia, and rarely pure Frontotemporal Dementia (FTD).
- ALS10: TAR DNA Binding Protein (TARDBP): The TARDBP gene encodes a DNA and RNA-binding protein called TDP-43. In healthy neurons, TDP-43 is located in the nucleus and regulates gene expression, RNA transcription, and splicing. Pathogenic mutations cause the TDP-43 protein to attain a hyper-phosphorylated and ubiquitinated form of RNA and DNA binding protein that is accumulated in neurons and spreads along the brain and spinal cord of ALS patients. Impaired TDP-43 functioning leads to neuronal degeneration with a limb or bulbar onset ALS. [11], [13] Other phenotypes associated with TARDBP mutations include Frontotemporal dementia (FTD), and ALS-FTD. ALS and FTD are heterogeneous in nature and share some clinical, neuropathological, and genetic features. Around 5–10% of ALS patients develop FTD. [14] Common features of ALS and FTD include cognitive deficits in attention, problem-solving, preservation of perception, and spatial functions.
5.1.2 Autosomal Recessive (AR)
In an autosomal recessive inheritance, the disease-associated gene must be inherited from both parents in order for an individual to develop the condition. ALSIN and OPTN (Optineurin) genes are common examples of AR inheritance in ALS.
- ALS2: ALSIN: ALS2 activates multiple proteins called GTPases that are essential for maintenance of motor neurons. [12] Mutations in ALS2 are responsible for autosomal recessive and early-onset forms of UMN diseases such as Juvenile amyotrophic lateral sclerosis (JALS). JALS is frequently caused by mutations in ALS2 genes and seldom caused by mutations in SETX (Senataxin), UBQLN2 (Ubiquilin2), and FUS genes. [15] JALS is a rare, severe motor neuron disease with a mean onset age of 6.5 years. It is characterized by limb and facial spasticity, gait, dysarthria, sensory disturbance, and bladder dysfunction.
- ALS12: Optineurin (OPTN): Mutations in OPTN have been reported in both SALS and FALS cases in either an autosomal dominant or recessive manner. OPTN gene regulates receptor-interacting kinase-1 enzyme (RIPK1), which plays a key role in inflammation and cell death. [16] Mutations in the OPTN gene causes neurotoxicity through dysmyelination and axonal degeneration.
5.1.3 X-Linked
In X-linked dominant inheritance, the gene responsible for the condition is located on the X chromosome. Mutation in either male and female copy of the gene can cause the disorder. Pathogenic variants in gene UBQLN2 (Ubiquilin 2) is related to X-linked dominant ALS.
- ALS 15/ Ubiquilin 2 (UBQLN2): Pathogenic variants in UBQLN2 encodes the ubiquitin-like protein ubiquitin-2, causing X-linked dominant ALS and ALS/dementia. Mutations in the UBQLN2 gene causes inclusions that can be seen in the spinal cord of affected patients. These inclusions are also seen for other ALS proteins such as TDP-43, FUS, and OPTN. ALS 15 can be an adult or juvenile onset disease. [12], [13]
5.2 Sporadic ALS (SALS)
Sporadic ALS is the most prevalent form of ALS consisting up to 90 to 95 percent of all ALS cases. [11] This type of ALS occurs sporadically with no known family history. Although the etiology of sporadic ALS is unknown, epidemiological data indicate that environmental & genetic factors contribute to its pathogenesis. Some of the risk factors that have increased ALS incidence are listed:
- Smoking: Cigarette smoking is the most consistent non-genetic risk factor for ALS. Cigarette smoke increases the probability of developing ALS through increased oxidative stress, inflammation, and neurotoxicity by heavy metals in cigarettes. Exhaled cigarette smoke has formaldehyde that is associated with higher mortality rates in ALS patients. [2]
- Physical Fitness: An increased risk of ALS is seen among athletes and individuals who engage in strenuous physical activities. Strenuous physical activity, repeated head injuries, use of illicit drugs, act as potential risk factors for ALS. Repeated head injuries cause chronic traumatic encephalopathy which has been proposed as a reason for ALS among professional athletes and military veterans. [2]
- Heavy Metals: Increased levels of lead in blood and bone were found to be associated with ALS. Lead and Manganese have neurotoxic properties that get accumulated in the nervous system causing damage to the neurons. [2] Other metals such as copper, aluminum, arsenic, uranium, cadmium, zinc, cobalt, and vanadium are found in significantly higher concentrations in the CSF of ALS patients when compared to healthy individuals.
- Radiation & Electromagnetic field: Magnetic fields, electrical fields, contact currents, micro-shocks, are often associated with occupational occupation exposing to involving low-frequency Electromagnetic field (EMF). Low-frequency electromagnetic waves produce oxidative stress that can disable the antioxidant properties of cells, leading to ALS. [17] However, none of the current studies report a conclusive connection between EMF exposure and oxidative stress in ALS development.
Several other factors such as pesticides, viruses, occupational workers (electrical workers, construction workers), dietary habits are proposed to be associated with ALS.
The etiology of sporadic ALS is complex. A combination of oxidative stress, mitochondrial dysfunction, glutamate excitotoxicity, inflammation, and apoptosis has been suggested as possible causes. Single-nucleotide polymorphisms (SNPs) in the paraoxonase gene cluster (PON) have been associated with sporadic ALS. PON enzymes are seen in insecticides, nerve gas agents, and in statin drugs. [18] Another example would be C9orF72 (Chromosome 9 open reading frame 72) which is a frequent cause of ALS, is responsible for about 10% of sporadic cases. [19] C9orf72 gene is also associated with familial ALS, Frontotemporal Dementia (FTD), and ALS with FTD. Few other examples of FALS genes mutations and variants occurring in sporadic ALS are ATXN2 gene, SETX gene, FUS gene. [19] These reports reinforce the concept that familial and sporadic ALS are not mutually exclusive categories but connected to each other.
- PATHOLOGICAL MECHANISM
Learning in depth about the molecular mechanisms for the degeneration of motor neurons in ALS can help in better understanding of the disease and can provide insight into developing newer strategies and treatments. This section provides a brief overview of the molecular and cellular mechanisms that have been proposed to contribute to ALS pathogenesis.
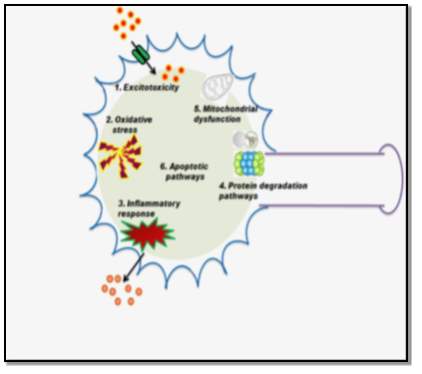
6.1 Glutamate Excitotoxicity
Glutamate is an important excitatory neurotransmitter in the central nervous system. During a normal neurotransmission process, glutamate is released into the synaptic cleft, where it activates postsynaptic receptors. This activity is regulated by transporter proteins, called excitatory amino acid transporters (EAATs). [20] Excessive activation of glutamate receptors and failure in clearing the neurotransmitter from the synaptic cleft can induce injury to neurons. This abnormal receptor activity leads to a massive influx of calcium that triggers apoptotic pathways causing motor neuron death and degeneration.
6.2 Mitochondrial Dysfunction
Mitochondria are the most important organelles for cellular respiration, energy production, calcium homeostasis, and apoptosis. They represent a primary site for intracellular production of reactive oxygen species (ROS), a major source of oxidative stress, that impairs the normal functioning of mitochondria. Hence, any structural alterations or mutations in mitochondria can lead to the pathogenesis of ALS. [21]
6.3 Oxidative Stress
Free radicals or ROS are natural byproducts of oxygen metabolism. Oxidative stress is caused when the production of ROS is greater than the capacity of cells to remove them. This excessive accumulation of ROS causes permanent damage to cell structures, DNA, and RNA causing motor neuron degeneration. SOD1 is an important protein to prevent oxidative damage in cells. [21] Mutation in the SOD1 gene causes disruption of cellular functions and cytotoxicity. This can be confirmed by testing the levels of oxidative stress. 3-nitrotyrosine (3-NT) is an established biomarker for oxidative stress and the levels of 3-NT is elevated in serum, urine, and CSF samples of ALS patients. [22]
6.4 Neuroinflammation
A familiar characteristic of ALS and other neurodegenerative diseases is the neuroinflammation. In the CNS, microglial cells are macrophages that act as the first line of defense against infections or injuries, thereby protecting motor neurons and astrocytes. Microglial cells have immunological properties, that can be either beneficial or harmful to motor neuron survival. As ALS progresses and the motor neuron damage worsens, the astrocytes and motor neurons release mutated SOD1 proteins that stimulate the activation of microglial cells. Activated microglial cells cause switch from neuroprotective and anti-inflammatory to a neurotoxic and pro-inflammatory phenotype. [21], [22]
- DIAGNOSIS
The early and accurate diagnosis of ALS can be challenging because of the complex and heterogeneous nature of ALS. There are no definitive diagnostic tests to prove ALS, hence differential diagnosis and investigations are conducted for an individual patient. This includes obtaining a thorough patient history, conducting neuroimaging scans, electromyography, laboratory tests, and genetic testing.
- Diagnosis Criteria
In the late 1990s, diagnostic criteria were developed to standardize diagnosis of ALS and research studies for clinical trials.
- The El Escorial criteria: As per this criterion, five categories of certainty of ALS were defined: clinically definite, clinically probable, clinically probable with laboratory support, clinically possible, and clinically suspected ALS. [23] The El Escorial criteria required clinical evidence in three of the four anatomic regions to be confirmed as definite ALS. These restrictions made it difficult to diagnose individuals with ALS. To improve the speed and certainty of diagnosis, laboratory tests were introduced as diagnostic tools to exclude differential diagnosis. This revised criterion was added to the El Escorial criteria and was renamed to the Airlie House Criteria in 1998. In 2008, neurophysiological measurements for LMN and UMN degeneration was incorporated, and it was known as Awaji-shima criteria. The Awaji criteria classifies the certainty level of ALS into three categories: Clinically definite ALS, Clinically probable ALS, and Clinically possible ALS. [23]
- The Japanese ALS severity classification: This categorization varies from El Escorial diagnostic criteria based on the functional severity of the patients. The Japanese ALS severity can be classified as;
Grade 1: Able to work or perform housework;
Grade 2: Independent living but unable to work;
Grade 3: Requiring assistance for eating, excretion, or ambulation;
Grade 4: Presence of respiratory insufficiency, difficulty in coughing out sputum, or dysphagia;
Grade 5: Using a tracheostomy tube, tube feeding, or tracheostomy positive-pressure ventilation.
- Differential Diagnosis
The primary diagnosis of ALS is conducted by clinical examination and series of diagnostic tests that help to exclude diseases that mimic ALS. Some of the conditions that mimic ALS are Cervical spondylotic myelopathy, Kennedy disease (KD), Multiple Sclerosis, Parkinson’s diseases, and Post-polio syndrome (PPS). The following table shows a list of differential diagnosis and clinical overlap with ALS; [24]
| Differential diagnosis of ALS | Clinical overlap with ALS | Diagnostic test to rule out |
| Kennedy syndrome | Progressive motor neuron degeneration | Genetic testing, blood test for identification of specific mutations |
| Huntington disease | Progressive motor disturbances and involuntary movements | Genetic testing |
| Post-polio progressive muscular atrophy | Double vision, droopy eyelids, muscle weakness | EMG, blood tests |
| Parkinson’s disease | Progressive motor dysfunction and bradykinesia | Combination of laboratory tests and neuroimaging |
| Multiple sclerosis | Sensory loss and muscle weakness | Neuroimaging and spinal tap |
For example, Kennedy disease (KD), known as spinobulbar muscular atrophy, is an X-linked disorder of brainstem and spinal cord. KD symptoms demonstrate slow progressive LMN signs in the bulbar region and proximal limbs, fasciculations, mild cognitive impairment, sensory disturbance, and gynecomastia. [24] In addition, increased creatine kinase (CK) levels and low amplitude of sensory nerve action can help to differentiate KD from ALS. Progression of KD is slower than that of typical ALS. To confirm this diagnosis, a genetic test for detection for KD is also required.
- Diagnostic Tests
Although the essential diagnostic criteria of ALS are defined by the El Escorial criteria, many misdiagnoses still occur. ALS mimic syndromes can be in terms of the anatomy, symptoms, or clinical manifestations. There is no single confirmatory test for ALS, but an extensive workup can help to rule out differential diagnosis. A comprehensive diagnostic workup includes most of the following procedures: [23]
- Electro-diagnostic test: Nerve Conduction studies and Needle Electromyography can detect the presence of fibrillation/fasciculations and sharp positive waves (together referred to as spontaneous activity). These tests can help in definitive diagnosis of ALS.
- Radiology tests: MRI for C-spine, lumbar spine, and thoracic spine.
- Laboratory test: CSF analysis, 24-hour urine collection for heavy metals, muscle biopsy, blood work including CBC, B12, folate, Creatine Kinase (CK), Serum protein electrophoresis (SPEP), Urine protein electrophoresis (UPEP). (CK, SPEP, and UPEP show elevated levels in ALS patients as compared to healthy individuals).
- Neurological test: Physical & mental health examination, cranial & motor nerves examination, sensory examination, gait, reflexes, etc.
- Genetic testing
A definitive diagnosis of ALS requires evidence of LMN and UMN degeneration, signs of progression, and spread of neurological symptoms within the anatomical region. The electrophysiological, laboratory, and neuroimaging results should not show evidence of any other pathological symptoms that mimic ALS.
- Functional Endpoints
Muscle strength and function are considered the most important endpoints that show effectiveness and consistency for any symptomatic treatment. For ALS, they can be assessed by;
- ALS Functional Rating Scale (ALSFRS):The Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) and the revised version that includes respiratory function (ALSFRS-R) is the most extensively used instrument to measure the efficacy of a drug in ALS clinical trials. The ALSFRS-R is an evaluation technique for monitoring the progression of disability in ALS patients based on twelve questions for fine motor, gross motor, bulbar, and respiratory functions. [25] Each question has five possible responses and each item is scored from 0 to 4 point range. Higher scores suggest greater functional ability.
- Percent Forced vital capacity (%FVC): %FVC is method for the assessment of Respiratory Function in ALS patients. A decrease in %FVC (not greater than 50%) is considered as a criterion for respiratory support. [25]
Other scales that measure functional disability are the Norris scale, the Appel Scale, and the Pinch grip strength method. However, the ALSFRS-R is the preferred scale. If it is not used as primary endpoint, it can be used as a secondary one.
- TREATMENT
Although pathological mechanisms have been explained, ALS remains incurable disease because of failure of clinical trials and lack of any effective therapy. The rapid advancement in genetic discoveries in ALS emphasizes the point that ALS is a multi-subtype syndrome rather than a single disease. This can be one of the reasons why many previous clinical trials have failed. This section will review the recent developments in therapeutic compounds and alternative therapies for ALS.
- Therapeutic Approaches in ALS
As the pathogenesis of ALS is complex, there is no effective treatment to cure ALS. Nevertheless, there are several therapeutic strategies can slow the progression of symptoms, prevent complications, and prolong survival.
- Radicava
The FDA approved Radicava in May 2017 in the United States, based on a six-month clinical trial conducted in Japan and granted it an Orphan drug designation. Radicava was discovered and developed by Mitsubishi Tanabe Pharma Corporation and will be commercialized in the United States by MT Pharma America. [26] It has been approved as a treatment option for ALS in Japan and South Korea. The drug is primarily known to slow the decline in physical function and delay the progression of ALS.
- Physical Properties
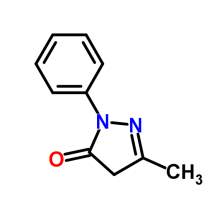 Edaravone is an active ingredient in Radicava, which is a member of the substituted 2-pyrazolin-5-one class. The chemical name of edaravone is 3-methyl-1-phenyl-2-pyrazolin-5-one. The molecular formula is C10H10N20 and the molecular weight is 174.20. Edaravone is a white crystalline powder and has a melting point of 129.7°C. It is soluble in acetic acid, ethanol, methanol, and slightly soluble in water. [27] Radicava injection supplied for intravenous infusion contains 30 mg edaravone in 100 mL isotonic, sterile, aqueous solution.
Edaravone is an active ingredient in Radicava, which is a member of the substituted 2-pyrazolin-5-one class. The chemical name of edaravone is 3-methyl-1-phenyl-2-pyrazolin-5-one. The molecular formula is C10H10N20 and the molecular weight is 174.20. Edaravone is a white crystalline powder and has a melting point of 129.7°C. It is soluble in acetic acid, ethanol, methanol, and slightly soluble in water. [27] Radicava injection supplied for intravenous infusion contains 30 mg edaravone in 100 mL isotonic, sterile, aqueous solution.
- Mechanism of Action
Radicava (Edaravone/MCI-186) is a neuroprotective drug that has properties of a free radical scavenger. Since oxidative stress is one of the major cause ALS, removal of free radicals may offer therapeutic benefits. Several free radical scavengers have been assessed for their efficacy, but only a few drugs have shown success in studies conducted. Edaravone is known to eliminate lipid peroxides and hydroxyl radicals and protects neurons from increased oxidative stress. [28] A reduced concentration of an oxidative stress biomarker, 3-nitrotyrosine, is seen in the cerebrospinal fluid. Edaravone can readily cross the blood-brain barrier (BBB), thereby explaining its efficacy while other scavengers have failed to demonstrate such effectiveness. [29] Thus, treatment with Edaravone slows the progression of functional motor disturbances in ALS patients
- Pharmacokinetics (PK) of Edaravone [30]
- Absorption– Edaravone is administered by IV infusion. The maximum plasma concentration of edaravone is reached by the end of infusion. Edaravone does not accumulate in plasma even after administration of multiple doses.
- Distribution– Edaravone is bound to human serum protein, albumin (92%), with no concentration dependence in the range of 0.1 to 50 micromol/L.
- Metabolism– Edaravone is metabolized into sulfate and glucuronide conjugates in the liver. These metabolites are not pharmacologically active and do not inhibit or induce isozymes. The mean terminal half-life of edaravone is about 4.5 to 6 hours. The half-life of its metabolites is 2 to 2.8 hours.
- Excretion– Approximately 70-90% of Edaravone is excreted in the urine as its glucuronide conjugate form and about 5-10% of the dose is recovered in the urine as sulfate conjugate form. Only about 1% or less form of the dose is detected in the urine as in an unchanged form.
- Toxicology Assessment [30, 31]
The toxicity potential of edaravone has not been assessed adequately. There is no reported evidence of carcinogenic abnormalities. Edaravone was also tested negative for bacterial reverse mutation in Chinese hamster lung chromosomal aberration (in vitro) and mouse micronucleus assays (in vivo). Intravenous administration of edaravone had no effect on fertility. However, disruption of the estrus cycle and irregular mating behavior was noted at the highest dose tested.
- Adverse Reactions [30, 31]
Some common adverse reactions that occurred in Edaravone treated patients were contusion, gait disturbance, and headaches. Adverse effects of edaravone were observed in embryo development studies that include decrease in fetal body weight, delays in markers of development, and increased mortality. There were a few deaths in edaravone controlled studies as well as in placebo-treated patients. These deaths were related to respiratory failure which is the most common cause of death in ALS.
- Specific Population Studies [30, 31]
Population PK analysis indicates that the pharmacokinetics of edaravone is not affected by gender, age, race, or weight. There is a fetal developmental risk associated with the use of edaravone in pregnant women. However, there is no adequate data to prove the presence of edaravone in human milk, effects of the drug on milk production, or on the breastfed infant. No safety concerns related to hepatic or renal impairment has been reported.
- Clinical Trials
The edaravone clinical development program for edaravone started in 2001for treatment of ALS in Japan. The ALS clinical trial program was conducted to explore efficacy and safety of edaravone and consisted of one Phase II and four Phase III studies. All the clinical trials were conducted in accordance with Good Clinical Practice and the guiding principles of the Declaration of Helsinki.
- Study 12 (MCI 186-12) [31]: Study 12 was a Phase II, open‐label, exploratory study in ALS patients. In this study, 5 patients were administered 6 cycles of edaravone 30 mg/day, and 14 patients were administered 6 cycles of edaravone 60 mg/day. The first cycle consisted of 2 weeks of daily infusion of the drug followed by 2 weeks without treatment. Based on results, efficacy was assessed for the 60 mg/day group and was selected as the dose to be tested in all Phase 3 studies. The primary endpoint was the change in the revised ALS functional rating scale (ALSFRS-R) that showed improved scores for patients on edaravone treatment.
- Study 16 (MCI 186-16) [31], [32]: Based on phase II preliminary results, phase III studies (MCI 186-16) were conducted to evaluate the efficacy and safety of edaravone at a dose of 60 mg per day. This was a multi-center, placebo-controlled, double-blind, parallel-group comparative study in ALS patients. This study enrolled 206 ALS patients of Grade 1 and 2 (Japanese ALS severity grade) and patients with definite ALS, probable ALS, or probable laboratory supported (ALS El Escorial Revised Airlie House criteria) within 3 years of screening. Patients were randomized to receive either edaravone or placebo for 24 weeks. The primary efficacy endpoint was the change in the ALSFRS‐R score from baseline to the end of 24 weeks. It was concluded that edaravone is effective in patients with mild ALS (Japanese ALS severity grade 1 & 2).
- Study 17 (MCI 186-17) [31], [33]: This study was a placebo-controlled extension of study 16, in which patients who received edaravone in Study 16 were re-randomized to receive edaravone or placebo, while patients who received placebo in study 16 were switched to edaravone. The primary efficacy endpoint was the change in ALSFRS‐R score. That study did not provide any useful efficacy information.
- Study 18 (MCI 186-18) [31], [34]: Thisphase III study was an exploratory, randomized, placebo-controlled study in patients with advanced ALS (Japanese ALS severity grade 3). The primary efficacy endpoint was the change in ALSFRS‐R score. This study yielded no significant difference in the scores between the treatment group and the placebo group. Study 18 demonstrated that edaravone lacks efficacy in patients with advanced ALS severity.
- Study19 (MCI 186-19) [31], [35]: Study 19 (MCI 186-19) was a second confirmatory Phase III study comprising two study periods; a randomized, placebo-controlled period of six cycles for 137 subjects and an active extension period of six cycles for a subset of ALS patients. This study design was similar to study 16 and used the same primary endpoint, i.e. change in ALSFRS‐R score at the end of 24 weeks. Secondary endpoints such as disease progression, percent FVC, Modified Norris Scale score, and Pinch grip strength were examined. The primary population used for the efficacy analysis was the Full analysis set (FAS) and Analysis of covariance was used for ALSFRS-R. The primary endpoint analysis showed a significant difference, favoring edaravone over the placebo group (ALSFRS‐R score change of ‐7.50 for placebo and ‐5.01 for edaravone, p=0.0013). Statistically, the rate of decline in physical function was by 33 percent or 2.49 ALSFRS-R points. [36] All the secondary endpoints demonstrated a nominally significant score that favored Edaravone. The level of CSF 3-Nitrotyrosine (a biomarker for oxidative stress) was lower in most of the patients on edaravone treatment group, suggesting that edaravone could protect neuronal cells from oxidative stress and delay progression.
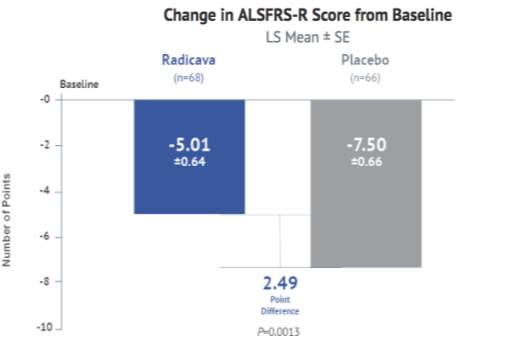
The following table provides a snapshot of the important clinical trial information for Edaravone.
Table 1: Studies in the ALS Development Program
| Study No. | Study Description | Study Design | No of Patients | Dosage Period |
| MCI186-12 | A Phase II exploratory study of edaravone in subjects with ALS | Open-labeled uncontrolled | 19 subjects
(30 mg group: 5 subjects, 60 mg group: 14 subjects) |
Cycle 1: Administration for 14 consecutive days, followed by a drug-free period of 2 weeks
Cycle 2 to 6: Administration of 5 days per week x 2 weeks, followed by a drug free period of 2 weeks |
| MCI186- 16 | A Phase III double-blind parallel-group study of edaravone for treatment of ALS (confirmatory study) | Randomized, double-blind, placebo-controlled, parallel-group, comparative | 206 subjects
(P group: 104subjects, E group: 102 subjects) |
Cycle 1: Administration for 14 consecutive days, followed by a drug-free period of 2 weeks
Cycle 2 to 6: Administration of 10 days over 2 weeks, followed by a drug free period of 2 weeks |
| MCI186- 17 | A Phase III double-blind parallel-group study of edaravone for treatment of ALS (extension study MCI186- 16) | Double-blind, placebo-controlled, parallel-group, comparative | 181 subjects
(EP group: 45 subjects, EE group: 48 subjects, PE group: 88 subjects) |
Cycle 7 to 15 (continued from MC1186-16): Administration of 10 days over 2 weeks, followed by a drug free period of 2 weeks
Cycle 13 to 15: Active treatment |
| MCI186- 18 | A Phase III double-blind parallel-group study of edaravone for treatment of ALS severity Grade 3 (exploratory study) | Randomized, double-blind, placebo-controlled, parallel-group, comparative, exploratory | 25 subjects
(P group: 12 subjects, E group: 13 subjects) |
Cycle 1: Administration for 14 consecutive days, followed by a drug—free period of 2 weeks
Cycle 2 to 6: Administration of 10 days per 2 weeks, followed by a drug free period of 2 weeks |
| MCI186- 19 | A Phase III double-blind parallel-group study of edaravone for treatment of ALS (second confirmatory study) | Randomized, double-blind, placebo-controlled, parallel-group, comparative with 6-month active treatment period | 137 subjects
(P group: 68 subjects, E group: 69 subjects) |
Cycle 1: Administration for 14 consecutive days, followed by a drug-free period of 2 weeks
Cycle 2 to 12: Administration for a total of 10 days over 2 weeks, followed by a drug free period of 2 weeks |
P group: placebo group E group: edaravone group
EP group: edaravone group in MCI 186-16 followed by placebo group in MCI186-17
EE group: edaravone group in MCI 186-16 followed by edaravone group in MCI186-17
PE group: placebo group in MCI 186-16 followed by edaravone group in MCI186-17
- Riluzole
Rilutek (riluzole) was the first drug approved by the FDA, in December 1995, to treat ALS. Rilutek is an oral formulation that acts to slow the progression of ALS symptoms and improve survival. The known mechanism of riluzole is to inhibit the production of glutamate and inactivate the voltage-dependent sodium channels thereby reducing glutamate toxicity in the cells. [22] The benefits of Riluzole can help to improve bulbar and limb function, but not for the muscle strength. Treatment with Riluzole can prolong survival but has minimal effect on the neurological function and has no effect on advanced stages of ALS.
- Masitinib
A French Biotech company named AB Science has revealed positive results for Phase III trial for masitinib in patients with ALS. Masitinib has proven to be beneficial in reducing neuroinflammation and slowing the progression of ALS. It is a tyrosine kinase inhibitor that targets mast cells, microglial cells, and resident macrophages by inhibiting the growth and migration of inflammatory cells within the central nervous system. As a part of the trial, 384 patients were recruited and masitinib was orally administered at 4.5 mg/kg/day as an add-on to riluzole for 48 weeks. [21], [37] This randomized, double-blind trial studied the efficacy and safety of a combination of masitinib and riluzole to that of a placebo and riluzole. Patients that were on masitinib and riluzole trial showed significant benefit as compared with placebo and riluzole. The primary endpoint of the trial showed success in the ALSFRS-R over the 48 weeks.
- Symptomatic Treatments
A team of specialized professionals such as neurologist, nutritionist, qualified nurses, speech therapist, psychologist, respiratory therapist can prove beneficial. Each individual may portray different manifestations of the disease and react differently to the prescribed drugs. The physician can gauge individual symptoms and suggest variable treatments to lower the risk of adverse side effects.
- Respiratory Management: Respiratory failure is a frequent feature of ALS and is roughly present in all cases at some stage of the illness. Death due to respiratory failure is caused due to fatigue and weakness of the diaphragm, coupled with respiratory muscle weakness, leading to poor lung functioning. The most commonly used measure for detecting respiratory decline is the examination of the patient’s Forced Vital Capacity (FVC). Lower FVC is associated with shorter survival. Therapeutic use of Noninvasive Ventilation slows the decline of FVC and prolongs survival in ALS patients. Invasive ventilation support like tracheostomy can be considered for patients who do not benefit from Noninvasive ventilation support. [38], [39]
- Spasticity & cramps: Spasticity or cramps can cause severe pain and discomfort. They are caused by involuntary muscle contractions that are prevalent in ALS. Spasticity treatments differ and may include physiotherapy and prescription medication. Often, warm bath or gentle massage can be helpful in relieving the discomfort associated with muscle cramps, twitching, and tightness. [39]
- Sialorrhea: Sialorrhea occurs because of weak the muscles in the mouth and throat. Treatments include suction of saliva from the salivary glands, or by invasive approaches such as injection of botulinum toxin or irradiating the salivary glands. Thick mucus production is often associated with sialorrhea and can be dealt with by humidification of air, increased fluid intake, or cough augmentation with respiratory therapy. [39]
- Pain: Pain in ALS can arise from muscle spasms, spasticity, and weakness. Reduced mobility can cause musculoskeletal pain. Common options include non-opioid analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids. NSAIDs inhibits the production of prostaglandins that cause pain, while opiates such as morphine imitate natural neuro-mediators and relieve pain. [21], [39]
- Sleep: ALS related symptoms such as dysphagia, anxiety, pain, depression, and disordered breathing can interfere with sleep patterns. Inability to change posture during sleep because of weakness, fasciculations, and muscle cramps may cause discomfort while sleeping. Sleep problems can be treated by the use of mild sedatives and Noninvasive Ventilation for respiratory issues.
- Loss of appetite: It is important to maintain weight for ALS affected individuals, as severe weight loss indicates muscle loss. Dysphagia is one of the main reasons of weight loss. As the muscles weaken, chewing, swallowing, eating, and drinking becomes difficult, less pleasurable, and time-consuming. Frequent small meals, rich in fat and protein are recommended. Adequate fluid intake aids in hydration, avoiding constipation, and thickened saliva. [39]
It is important to have multidisciplinary management system in place for managing patients with ALS to enhance health care delivery, improve the quality of life, and prolong survival. Important issues such as the course of the disease, respiratory management, and other critical decisions should be discussed with patients and relatives that may arise during the later stages of ALS.
- Stem Cell Therapy
Stem cell therapy is an emerging and promising potential treatment option for ALS. Stem cells can support dying motor neurons by reducing inflammation, releasing growth factors, and other potential mechanisms. Different types of stem cells have been explored as possibilities for treating ALS, including embryonic stem cells, neural stem cells, mesenchymal stem cells, and induced pluripotent stem cells. Phase II clinical trials have been completed for mesenchymal stem cells derived from bone marrow of ALS affected individuals. [22] These cells are propagated ex vivo and induced to secrete neurotrophic factors (MSC-NTF). They are again transplanted back into the ALS patient’s sites of damage, the spinal cord, and the muscles. Delivery of NTF’s to the immediate environment of affected neurons improves their survival and thus slows down disease progression and symptoms. Recently, Cedars-Sinai regenerative medicine received FDA approval to test a novel combination stem cell and gene therapy for ALS patients. [40]
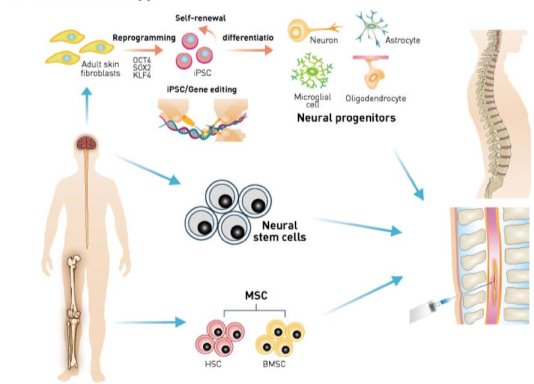
- Biomarkers
Biomarkers are biological systems that help to identify the presence of a disease, rate of progression of a disease, or the effectiveness of a therapeutic invention. Since ALS is difficult to diagnose, an ideal biomarker should exhibit high sensitivity and specificity to distinguish ALS from disease mimics and other neurologic diseases. Significant progress has been made for cerebrospinal fluid, plasma, and serum biomarkers, whereas urine and saliva biomarkers are still in the early stages of development. [22]
- Genetic Counseling
The genetic landscape of ALS is complex and difficult to understand. Patients with amyotrophic lateral sclerosis often have questions about why they acquired the disease and the probability of other family members being affected with the same. Providing information to the affected families based on the nature, inheritance, and implications of ALS can help them make informed medical and personal decisions. [22]
- SUMMARY
Amyotrophic lateral sclerosis is a fatal neurodegenerative disease affecting the nervous system. It causes progressive and cumulative physical disabilities in patients and eventually death due to respiratory muscle failure. ALS can result from various causes: genetic, environmental, or a combination of both. Around 25 genes are associated with ALS and the most common mutations are seen in SOD1, TARDBP, FUS and C9orf72 genes. The complexity and heterogeneous nature of ALS make early and accurate diagnosis very challenging. Recently, FDA approved Radicava that prolongs survival and aids in improving the quality of life for ALS patients. Radicava acts as a ‘free radical scavenger’ and helps the body to eliminate excess free radicals, and prevent cell damage. Currently, we rely on a multidisciplinary approach to manage and care for patients suffering from ALS. Multidisciplinary management helps patients to optimize healthcare facilities, ease communication between family members, and thus improve the quality of life.
- CONCLUSION
Over the last decade awareness of ALS has significantly increased. Unraveling the ALS genome, better drug delivery system, and the application of pharmacogenomics and stem cell therapy are some future avenues that are likely to bring success.
The importance of the MCI-186 study 19 is due to the incorporation of the ALSFRS-R, the most widely applied rating scale in clinical practice and clinical trials as a primary or secondary outcome measure. Other ALS trials using the ALSFRS-R have not been able to demonstrate any significant change in the slope of this measure. However, it is also necessary to understand that Edaravone has been approved by the FDA based on a 6-month trial tested only on Japanese patients. There is no concrete information relating to the longer-term effect of Edaravone on safety, function, or overall survival. Lastly, the MCI-186 study 19 selected a clinically defined subgroup of ALS patients (Japanese grade 1 & 2, or The El Escorial criteria) as part of the clinical trial design, a concept being more widely adopted by the ALS community to develop precision medicine. However, for future clinical trials, identifying the specific subgroups will be challenging.
Non-pharmacological strategies such as Stem cell therapy, Gene therapy, Immunotherapy, Vaccines can be used to modify, slow down, or halt the progression of the disease. Stem cells can improve neuromuscular functioning by providing protective factors to surrounding cells to inhibit inflammation, or by replacing injured cells. Thus, stem cells can help in correcting the mutations in patients with familial ALS as well as sporadic ALS.
A plethora of evidence-based guidelines should be compiled into internationally agreed guidelines for uniform practice. Current discoveries have helped in slowing down the progression of the disease, but the future treatments should aim at preventing neuronal damage right from the initial onset of the disease. Hopefully, the success of the edaravone development program is the first of many new therapies for the disease.
“Let us keep looking, in spite of everything. Let us keep searching. It is indeed the best method of finding, and perhaps thanks to our efforts, the verdict we will give such a patient tomorrow will not be the same we must give this man today.”
Charcot (1889)
LIST OF ABBREVIATIONS
| AD | Autosomal Dominant | JALS | Juvenile Amyotrophic Lateral Sclerosis |
| ALS | Amyotrophic Lateral Sclerosis | KD | Kennedy Disease |
| ALSA | ALS Association | LMN | Lower Motor Neurons |
| ALSFRS | Amyotrophic Lateral Sclerosis Functional Rating Scale | MSC-NTF | Mesenchymal Stem Cells- Neurotrophic Factors |
| ALSFRS-R | Revised ALSFRS that includes respiratory function | NSAID | Non-Steroidal Anti-Inflammatory Drug |
| ALSIN | Amyotrophic Lateral Sclerosis 2 | NT | Nitrotyrosine |
| AR | Autosomal Recessive | OPTN | Optineurin |
| ATXN2 | Ataxin 2 | PK | Pharmacokinetics |
| BBB | Blood Brain Barrier | PON | Paraoxonase |
| BMAA | β–methylamino-L-alanine | PPS | Post-polio syndrome |
| C9orf72 | Chromosome 9 open reading frame 72 | RIPK1 | Receptor-Interacting Kinase 1 |
| CK | Creatine Kinase | RNA | Ribonucleic acid |
| CNS | Central Nervous System | ROS | Reactive Oxygen Species |
| CSF | Cerebrospinal Fluid | SALS | Sporadic ALS |
| DNA | Deoxyribonucleic acid | SETX | Senataxin |
| EAAT | Excitatory Amino Acid Transporters | SNP | Single-nucleotide polymorphisms |
| EMF | Electromagnetic field | SOD1 | Superoxide Dismutase 1 |
| EMG | Electromyography | SPEP | Serum protein electrophoresis |
| FALS | Familial ALS | TARDBP | TAR DNA Binding Protein |
| FAS | Full analysis set | TDP-43 | TAR DNA-binding protein 43 |
| FTD | Frontotemporal Dementia | UBQLN2 | Ubiquilin 2 |
| FUS | Fused In Sarcoma | UMN | Upper Motor Neurons |
| FVC | Forced Vital Capacity | UPEP | Urine Protein Electrophoresis |
REFERENCES
- The ALS Association. Retrieved October 15, 2017, from http://www.alsa.org/about-als/what-is-als.html?referrer=https://www.google.com/
- Ingre, C., Roos, P. M., Piehl, F., Kamel, F., & Fang, F. (2015). Risk factors for amyotrophic lateral sclerosis. Clinical Epidemiology, 7, 181–193. http://doi.org/10.2147/CLEP.S37505
- Finger, S., Boller, F., & Tyler, K. L. (2009). History of Neurology. Elsevier.
- Perez, S., The Ice Bucket Challenge, By the Numbers. Retrieved November 1, 2017, from http://social.techcrunch.com/2014/09/03/the-ice-bucket-challenge-by-the-numbers/
- The ALS Association. Retrieved November 1, 2017, from http://www.alsa.org/fight-als/ibc-progress.html
- Arthur, K. C., Calvo, A., Price, T. R., Geiger, J. T., Chiò, A., & Traynor, B. J. (2016). Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nature Communications, 7, 12408. http://doi.org/10.1038/ncomms12408
- Prevalence of Amyotrophic Lateral Sclerosis – United States, 2010-2011. (2015, January). Retrieved November 1, 2017, from https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6307_brief.htm
- Mehta, P., Antao, V., Kaye, W., Sanchez, M., Williamson, D., Bryan, L., … Horton, K. (2014). Prevalence of amyotrophic lateral sclerosis-United States, 2010-2011. Morbidity and Mortality Weekly Report, 63(7). Retrieved from https://www.cdc.gov/mmwr/pdf/ss/ss6307.pdf
- Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., … Zoing, M. C. (2011). Amyotrophic lateral sclerosis. The Lancet, 377(9769), 942–955. https://doi.org/10.1016/S0140-6736(10)61156-7
- Kinsley, L., & Siddique, T. (1993). Amyotrophic Lateral Sclerosis Overview. In M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. Bean, H. C. Mefford, … N. Ledbetter (Eds.), GeneReviews(®). Seattle (WA): University of Washington, Seattle. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK1450/
- Boylan, K. (2015). Familial ALS. Neurologic Clinics, 33(4), 807–830. http://doi.org/10.1016/j.ncl.2015.07.001
- Li, H.-F., & Wu, Z.-Y. (2016). Genotype-phenotype correlations of amyotrophic lateral sclerosis. Translational Neurodegeneration, 5, 3. http://doi.org/10.1186/s40035-016-0050-8
- Chen, S., Sayana, P., Zhang, X., & Le, W. (2013). Genetics of amyotrophic lateral sclerosis: an update. Molecular Neurodegeneration, 8, 28. http://doi.org/10.1186/1750-1326-8-28
- Ferrari, R., Kapogiannis, D., Huey, E. D., & Momeni, P. (2011). FTD and ALS: a tale of two diseases. Current Alzheimer Research, 8(3), 273–294. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3801195/
- Juvenile amyotrophic lateral sclerosis | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Retrieved November 1, 2017, from https://rarediseases.info.nih.gov/diseases/11901/juvenile-amyotrophic-lateral-sclerosis
- Ito, Y., Ofengeim, D., Najafov, A., Das, S., Saberi, S., Li, Y., … Yuan, J. (2016). RIPK1 Mediates Axonal Degeneration By Promoting Inflammation and Necroptosis in ALS. Science (New York, N.Y.), 353(6299), 603–608. http://doi.org/10.1126/science.aaf6803
- Kheifets, L., Bowman, J. D., Checkoway, H., Feychting, M., Harrington, J. M., Kavet, R., … van Wijngaarden, E. (2009). Future needs of occupational epidemiology of extremely low frequency electric and magnetic fields: review and recommendations. Occupational and Environmental Medicine, 66(2), 72–80. https://doi.org/10.1136/oem.2007.037994
- Saeed, M., Siddique, N., Hung, W. Y., Usacheva, E., Liu, E., Sufit, R. L., … Siddique, T. (2006). Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology, 67(5), 771–776. https://doi.org/10.1212/01.wnl.0000227187.52002.88
- Martin, S., Al Khleifat, A., & Al-Chalabi, A. (2017). What causes amyotrophic lateral sclerosis? F1000Research, 6, 371. http://doi.org/10.12688/f1000research.10476.1
- Shaw, P. J., & Ince, P. G. (1997). Glutamate, excitotoxicity and amyotrophic lateral sclerosis. Journal of Neurology, 244 Suppl 2, S3-14. https://doi.org/10.1007/BF03160574
- Kumar, V., Islam, A., Hassan, M. I., & Ahmad, F. (2016). Therapeutic progress in amyotrophic lateral sclerosis-beginning to learning. European Journal of Medicinal Chemistry, 121(Supplement C), 903–917. https://doi.org/10.1016/j.ejmech.2016.06.017
- Bonafede, R., & Mariotti, R. (2017). ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Frontiers in Cellular Neuroscience, 11. https://doi.org/10.3389/fncel.2017.00080
- Joyce, N. C., & Carter, G. T. (2013). Electrodiagnosis in Amyotrophic Lateral Sclerosis. PM & R: The Journal of Injury, Function, and Rehabilitation, 5(5 0), S89–S95. https://doi.org/10.1016/j.pmrj.2013.03.020
- Turner, M. R., & Talbot, K. (2013). Mimics and chameleons in motor neurone disease. Practical Neurology, practneurol-2013-000557. https://doi.org/10.1136/practneurol-2013-000557
- Paganoni, S., Cudkowicz, M., & Berry, J. D. (2014). Outcome measures in amyotrophic lateral sclerosis clinical trials. Clinical Investigation, 4(7), 605–618.
- Press Announcements – FDA approves drug to treat ALS [WebContent]. Retrieved November 1, 2017, from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm557102.htm
- Pubchem. edaravone. Retrieved November 1, 2017, from https://pubchem.ncbi.nlm.nih.gov/compound/4021
- Watanabe, T., Tanaka, M., Watanabe, K., Takamatsu, Y., & Tobe, A. (2004). [Research and development of the free radical scavenger edaravone as a neuroprotectant]. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan, 124(3), 99–111.
- Kikuchi, K., Miura, N., Morimoto, Y., Ito, T., Tancharoen, S., Miyata, K., … Kawahara, K. (2011). Beneficial Effects of the Free Radical Scavenger Edaravone (Radicut) in Neurologic Diseases. Journal of Neurology & Neurophysiology, 0(0). https://doi.org/10.4172/2155-9562.S1-001
- Radicava (edaravone injection), for intravenous use. (2017, June). Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209176lbl.pdf
- Summary Review of Edaravone.pdf. (2017, June). Retrieved November 1, 2017, from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209176Orig1s000SumR.pdf
- ClinicalTrials.gov [Internet]. Identifier NCT00330681, Efficacy and Safety Study of MCI-186 for Treatment of Amyotrophic Lateral Sclerosis (ALS) – Retrieved November 1, 2017, from https://clinicaltrials.gov/ct2/show/NCT00330681
- ClinicalTrials.gov [Internet]. Identifier NCT00424463, Expanded Controlled Study of Safety and Efficacy of MCI-186 in Patients With Amyotrophic Lateral Sclerosis (ALS) – Retrieved November 1, 2017, from https://clinicaltrials.gov/ct2/show/NCT00424463
- ClinicalTrials.gov [Internet]. Identifier NCT00415519, Efficacy and Safety Study of MCI-186 for Treatment of Amyotrophic Lateral Sclerosis (ALS) Who Met Severity Classification III. Retrieved October 20, 2017, from https://clinicaltrials.gov/ct2/show/record/NCT00415519
- ClinicalTrials.gov [Internet]. Identifier NCT01492686, Phase 3 Study of MCI-186 for Treatment of Amyotrophic Lateral Sclerosis – Retrieved November 1, 2017, from https://clinicaltrials.gov/ct2/show/NCT01492686
- Radicava Product Fact Sheet pdf. (2017, August). Retrieved November 1, 2017, from https://www.mt-pharma-america.com/wp-content/uploads/2017/08/RADICAVA_Product_Fact_Sheet_8.22.17.pdf
- Petrov, D., Mansfield, C., Moussy, A., & Hermine, O. (2017). ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment? Frontiers in Aging Neuroscience, 9. https://doi.org/10.3389/fnagi.2017.00068
- Hardiman, O. (2011). Management of respiratory symptoms in ALS. Journal of Neurology, 258(3), 359–365. https://doi.org/10.1007/s00415-010-5830-y
- Dorst, J., Ludolph, A. C., & Huebers, A. (2017). Disease-modifying and symptomatic treatment of amyotrophic lateral sclerosis. Therapeutic Advances in Neurological Disorders, 1756285617734734.
- Cedars-Sinai Receives Approval to Test Novel Combined Stem Cell and Gene Therapy for ALS Patients. (2016, October). Retrieved October 10, 2017, from https://www.cedars-sinai.edu/About-Us/News/News-Releases-2016/Cedars-Sinai-Receives-Approval-to-Test-Novel-Combined-Stem-Cell-and-Gene-Therapy-for-ALS-Patients.aspx
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




