The Age-Associated Deterioration in Muscle Performance
Info: 7990 words (32 pages) Dissertation
Published: 15th Feb 2022
Tagged: PhysiologyMedicine
Introduction
The population of Canada is continually growing older with 16.9% of the population being 65 years and older in 2016 (“The Daily — Age and sex, and type of dwelling data: Key results from the 2016 Census”, 2018). This group of individuals are projected to represent 23-25% of the population by the year 2036 (“Population Projections for Canada, Provinces and Territories: Highlights”, 2018). It is well established that with aging, muscle performance declines. As a result, physical capacities become more limited and this increases the likelihood of injuries and diseases. These issues affect the individual, their family, and the health care system. Therefore, it is critical to determine which factors can be modified to ensure improved physical function and ability.
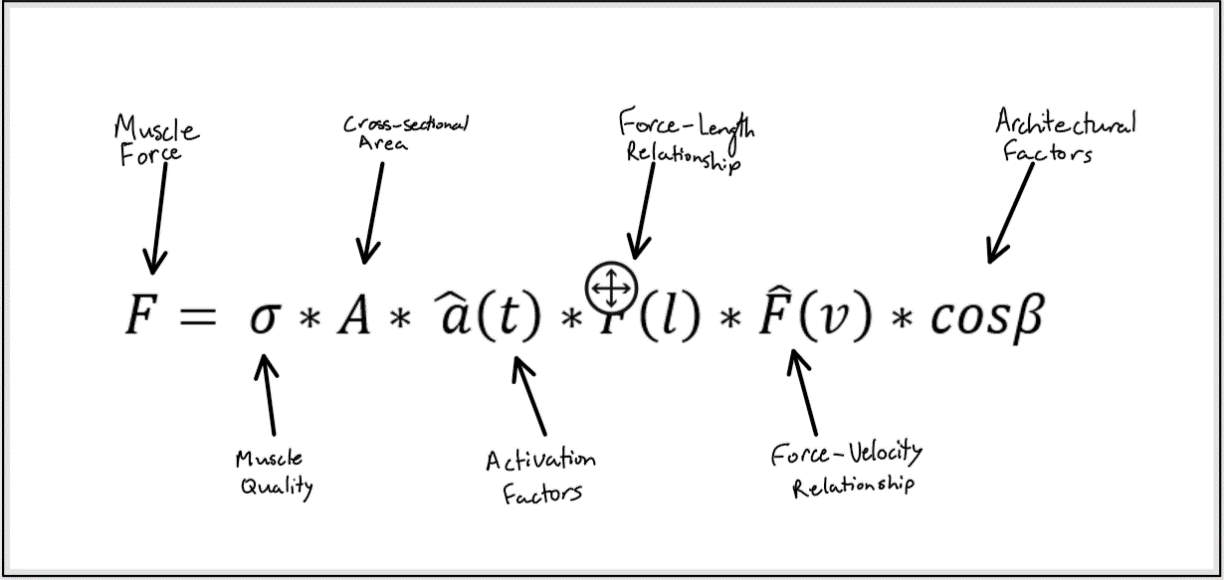
Figure 1: The factors that affect the amount of force produce by a muscle.
Muscle performance is determined by the force and power that a muscle can generate. There are multiple factors that affect the amount of force a muscle produces, outlined in figure 1. These same factors also affect muscle power since power is the product of force and velocity. Interestingly, power and force do not always decline to the same extent with aging which will be discussed later. The first factor we will consider in this paper is muscle quality. Muscle quality is the amount of force a muscle produces per unit of muscle cross-sectional area (Newman et al., 2003; Delmonico et al. 2009).
Several studies have demonstrated the importance of muscle quality on physical function and ability. Muscle quality is known to decline with age (Miljkovic, Lim, Miljkovic, & Frontera, 2015). Thus, muscle quality may be an important modifiable factor that may allow us to improve the physical functioning of older adults. Next, we will discuss how muscle cross-sectional area decreases with age. Then we will turn our attention to the changes in the force-length and force-velocity relationships that occur with age. Lastly, we will explore the architectural changes such as decreased pennation angle and shortened fascicle lengths that occur within aging muscle.
The deterioration in muscle performance that occurs with age influences the daily lives of older adults and their general well-being. Muscle performance is a major factor in frailty in older adults. Declines in muscle performance increase the risk of falls as the antigravity muscles are weaker and thus cannot maintain balance as effectively. Furthermore, the reduction in muscle power makes simple tasks such as sit-to-stand and walking up the stairs laborious (Siparsky, Kirkendall, & Garrett, 2014). Additionally, it results in decreased walking speeds which is positively associated with increased risk of mortality. It is critical to understand the factors that affect muscle performance and how these factors change with age so that they may be used in developing therapies to impede/decelerate the reductions in muscle performance. These therapies would be of utmost importance in improving the quality of life for older adults.
Changes in Muscle Quality with Aging
Muscle Fiber Type Transformation
The muscle fiber types that make up muscle and their relative contributions is an important determinant of muscle quality. There are three types of muscle fibers: Type I (slow oxidative), Type IIa (slow oxidative glycolytic), and Type IIb (fast glycolytic). These muscle fibers differ in their velocity of shortening, myosin heavy chain content (MHC), force of contraction and fatigability which is determined by the energy system they rely on. The classification of muscle fibers is based on the MHC isoform which they possess. The MHC isoform determines their shortening velocity. Type I muscle fibers are slow-contracting and produce low force of contraction, but they are fatigue resistant. Type IIa muscle fibers are fast-contracting with an intermediate force of contraction and are also fatigue resistant. Type IIb fibers are fast-contracting and produce high force of contraction, but fatigue very rapidly.
Since type I and type IIa fibers have a high density of mitochondria, myoglobin, and capillary content, they can sustain contractions for longer durations by generating ATP through aerobic cellular respiration. On the other hand, type IIb fibers have less intracellular myoglobin and mitochondria. Thus, they must rely on anaerobic glycolysis to produce ATP. Anaerobic glycolysis produces less ATP and as a result these muscle fibers deplete their energy reserve at a faster rate. Table 1 shows the general characteristics of the three muscle fiber types.
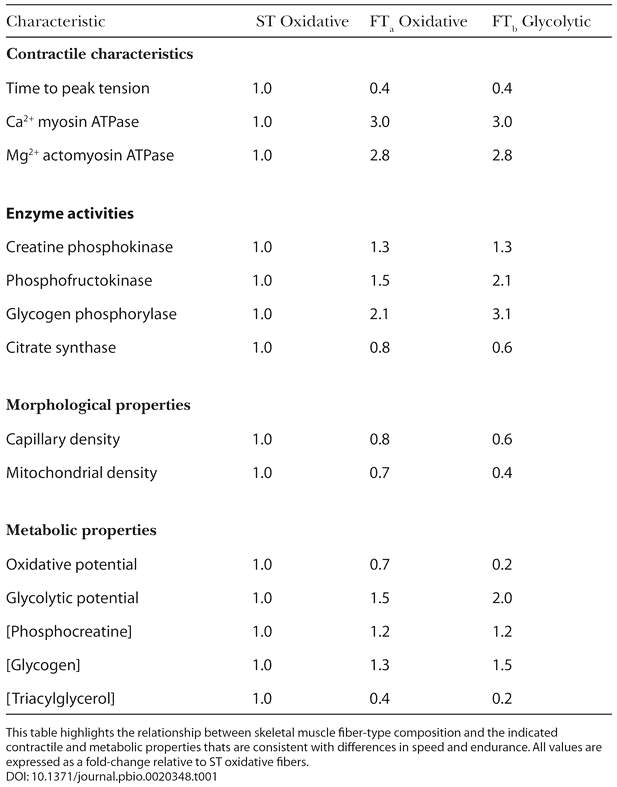
Table 1: This table shows the contractile, morphological, and metabolic properties of muscle.
Proctor, Sinning & Sieck (1995) investigated the relationship between aging and the fiber type composition of muscle. In this study they compared younger (21-30 year old) and older (52-61 year old) healthy adult men with normal levels of fitness. Additionally, they compared younger and older endurance trained men. Their findings showed that the older groups had smaller type IIa and type IIb fibers and a lower number of capillaries associated with these fibers when compared to the younger groups. Furthermore, they found that these reductions in cross-sectional area and capillary contacts were independent of training status.
Type I muscle fiber cross-sectional was not affected by age but was observed to be larger in the trained groups. The results of this experiment demonstrated that with aging there is a fast-to-slow fiber type shift and that endurance training does not offset this change. Thus, for older adults, a given muscle will have less type II fibers per cross-sectional area of muscle and as a result produce less force per cross-sectional area of muscle. This means that the muscle quality has decreased since muscle quality is defined as muscle force produced per cross-sectional area of muscle. Furthermore, muscle power also decreases because power is the product of force and velocity. Thus, as force decreases, power also decreases. Additionally, other studies have shown that the ability of muscle to hypertrophy is decreased with age (Welle, Totterman & Thornton, 1996).
Welle, Totterman & Thornton (1996) conducted a study to determine the effect of age on muscle hypertrophy. They hypothesized that the older adults (62-72 years old) in their experiment would show less hypertrophy than the young adults (22-31 years old). The subjects performed elbow flexion, knee extension, knee flexion, and lateral pulldown exercises 3 times a week for a period of 3 months. Prior to the exercise regimen, the younger and older adults had no significant differences in the cross-sectional area of their elbow and knee flexor muscles. After 3 months of training, the younger age group had significantly higher increases in the cross-sectional area of their elbow and knee flexors. Knee flexor cross-sectional area increased by 1 +/- 2% and 8 +/- 2% for the old and young groups respectively (p < 0.01). Elbow flexor cross-sectional area increased by 9 +/- 4% and 22 +/- 4% for the old and young group respectively (p < 0.05). The results of this study are shown in figure 2.
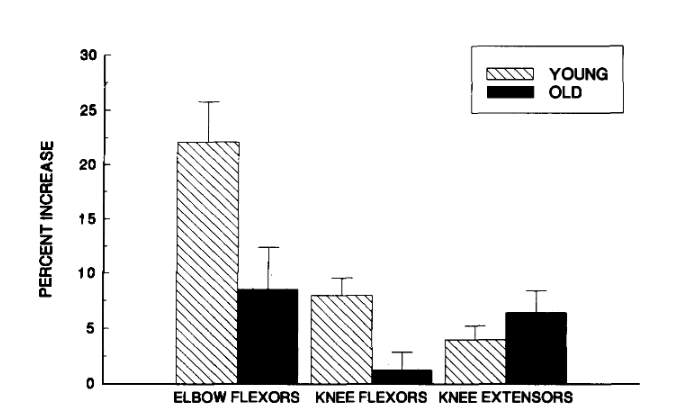
Figure 2: Mean percent increase in cross-sectional area (CSA) of elbow flexor, knee extensor, and knee flexor muscles after a 3-month resistance training program. Error bars are one standard error of the mean.
The heterogeneity of satellite cells is believed to be the reason for the differences in regenerative capacity of muscles between older and younger adults (Ciciliot, Rossi, Dyar, Blaauw & Schiaffino, 2013). Thus, with aging there is a fast-to-slow fiber type shift and there are changes in the muscle properties such that when younger and older adults undergo similar training, older adults show less hypertrophy.
Increased Intramuscular Fat with Age
It has been shown by several researchers that adipose tissue accumulates in skeletal muscle with age. Fat accumulates both within and outside of muscle fibers. This accumulated fat in muscle is referred to as intramuscular fat. As intramuscular fat content increases, there is a decrease in muscle quality because there is a decrease in myofibrillar content within a given muscle. Thus, intramuscular fat is inversely associated with muscle performance and positively associated with physical dysfunction.
Kent-Braun, V. Ng, and Young (2000) investigated the impact of age, gender, and physical activity on skeletal muscle composition. They studied 23 young (26 – 44 years old) and 21 older (65 – 83 years old) adults who were free of any debilitating diseases. Magnetic resonance imaging was used to determine the contractile and non-contractile content of the ankle dorsiflexor muscles. They found that the younger adults had significantly larger contractile areas than the older adults (p < 0.01). Additionally, they found that older adults had significantly larger absolute (cm2) and relative non-contractile areas compared to younger adults (p < 0.001). However, there was no significant difference in the total compartment area between the age groups.
These results indicate that muscle quality decreases with age because for a given area of muscle, older adults had less contractile content. The contractile component of muscle is responsible for generating force and power. The non-contractile component of muscle includes intramuscular fat which was found to increase with age and deteriorate muscle quality since it does not contribute to the force or power generated by muscle.
This decrease in muscle power that results from increased intramuscular fat can be expected to cause problems with activities that require large amounts of power such as walking. Unsurprisingly, Visser et al. (2005) found that there was an association between intramuscular fat and increased risk of mobility limitations. Additionally, other studies have found that increased intramuscular fat is associated with slower walking speeds in both males and females and lower grip strength in men (Therkelsen, Pedley, Hoffmann, Fox & Murabito, 2016). These findings indicate that increased intramuscular fat with age causes muscle performance to decline. There is a decrease in both muscle power (declines in walking speed) and muscle strength (lower grip strength).
It is very difficult to experimentally determine the effect of intramuscular fat on muscle performance (Rahemi, Nigam & Wakeling, 2015). The reason for this is two-fold. Firstly, muscle forces cannot be directly measured and secondly it is not possible to experimentally alter the intramuscular fat in subjects. Rahemi, Nigam, and Wakeling (2015) used a three-dimensional finite-element model to investigate the effect of intramuscular fat on muscle performance. They found that fatty models had lower force production than lean models. Since fat is a stiffer material than muscle, its infiltration in muscle results in an overall stiffer base material. When a muscle shortens, its volume can be assumed to stay the same. If the volume stays the same, then the muscle must bulge transversely to maintain its original volume. However, when the base material stiffness increases, it resists muscle fiber shortening and muscle bulging. Therefore, the muscle must do internal work to bulge transversely and this takes away from the force the muscle can produce to do external work. Hodgson et al. (2012) utilized a finite-element model and found that a stiffer base material resulted in lower muscle forces. Their results were consistent with Rahemi, Nigam, and Wakeling (2015). However, Hodgson et al. (2012) used a two-dimensional model and did not consider transverse bulging.
Changes in Cross-sectional Area of Muscle with Aging
The cross-sectional area of skeletal muscle decreases with age and results in reduced muscle strength and contributes to loss of independence, and higher risk for injuries due to falls (Marcell, 2003). This age-related decrease in skeletal muscle mass is referred to as sarcopenia. It important to understand that muscle cross-sectional area does not decrease uniformly among all fiber types. Researchers have consistently found that the number and size of type II fibers decrease with age and contribute to the lower muscle mass (Verdijk et al., 2007). However, type I fiber size is mostly unaffected and does not contribute significantly to the decrease in muscle mass associated with age (Verdijk et al., 2007; Janssen, Heymsfield, Wang & Ross, 2000; Jankowski, 2008).
Nilwik et al. (2013) conducted a study to determine the changes in muscle fiber size that occur with age and how these muscle fibers would change after 6 months of resistance training. The subjects of this study were 25 young (23 ± 1 years old) and 26 older (71 ± 1 years old) healthy adult men. The site of measurement was the quadriceps muscle. The cross-sectional area of the quadriceps muscle was significantly higher in the young men compared to the old men (80 ± 2 cm2 vs 68 ± 2 cm2 respectively; p < 0.001).
The older adults had significantly smaller type II muscle fibers when compared to the young men (p < 0.001). However, the differences in the size of type I muscle fibers was insignificant. They found that the differences in the quadriceps cross-sectional area for the two age groups was completely explained by the differences in the type II muscle fiber size. After 6 months of resistance training the elderly experienced a significant increase in the type II muscle fiber size (p < 0.01). Currently, the specific mechanisms that result in sarcopenia have not been determined. However, it has been hypothesized that satellite cells may play an important role in the loss of skeletal muscle.
Satellite cells are important for myofiber repair, growth, and maintenance (Verdijk et al., 2009). Since satellite cells maintain skeletal muscle mass, it has been hypothesized that in sarcopenia there may be a decrease in the number of satellite cells or that their ability to divide or function is altered (Verdijk et al., 2007). However, studies have been inconsistent in their findings with some noting that there is a lower number of satellite cells per muscle fiber in the elderly (Renault, Thorne, Eriksson, Butler-Browne & Mouly, 2002), and others not finding any significant differences (Dreyer, Blanco, Sattler, Schroeder & Wiswell, 2006).
Verdijk et al. (2007) speculated that the reduction in type II muscle fiber size could be associated with a reduction in the number of satellite cells for that specific muscle fiber type. They studied the muscle fiber characteristics and fiber type specific satellite cell content of the vastus lateralis muscles of eight young (20 ± 1 years old) and eight older (76 ± 1 years old) healthy adult men. They found that the type II muscle fiber cross-sectional area of the older males (4,451 ± 396 μm²) was significantly smaller than the younger adult men (6,126 ± 393 μm², p < 0.05).
This finding is consistent with what many other studies have found. The number of satellite cells per type II muscle fiber was significantly lower for the older adults (p < 0.05) There were no significant differences in the type I muscle fiber cross-sectional area for the two age groups. In addition, there was no significant difference in the satellite cell content of the type I muscle fibers for the two age groups. Thus, this study demonstrated that the type II muscle fibers atrophy with age due to a loss of satellite cell content, but this reduction in satellite cell content is not seen for the type I muscle fibers. Therefore, it could be inferred that with age there is no significant change in the type I fiber cross-sectional area because the number of type I muscle fiber-specific satellite cells does not decrease significantly.
Although, it is known that skeletal muscle mass decreases with age, it is important to quantify how this change affects the muscle performance of older individuals. Maden-Wilkinson, McPhee, Jones, and Degens (2015) conducted a study to investigate the relationship between muscle size, force, and power (Maden-Wilkinson, McPhee, Jones & Degens, 2015). The participants of the study were young (21 female, 28 males) and older (35 female, 31 male) adults who were healthy. The participant characteristics are shown in table 2.
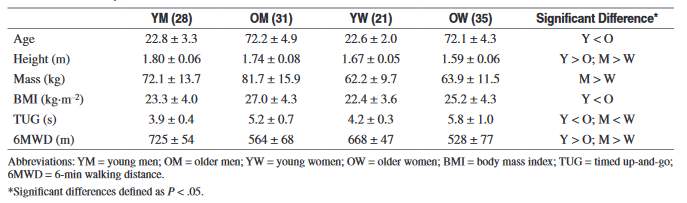
Table 2: The characteristics of the participants
The knee extensor maximum voluntary contraction (MVC) of the younger men and women were significantly higher than the older participants (p < 0.0005). The younger individuals’ MVC was about 1.66 times greater than the older participants. In addition, the younger participants generated significantly more power than the older participants (p < 0.0005). They generated more power regardless of whether power was expressed in terms of absolute power or relative power according to body mass.
Factors Affecting the Force Length Relationship with Aging
Decreased Tendon Stiffness
Tendons are bands of connective tissue which are composed of collagen. They join muscles to bones. The function of a tendon is to transmit force produced by muscles to bones to cause movement of the skeleton. Aging may cause changes in the mechanical properties of tendons. These changes could in turn affect the force-length and force-velocity curves of sarcomeres (Csapo, Malis, Hodgson & Sinha, 2014). Stenroth, Peltonen, Cronin and Finni (2012), studied the effect of age on the stiffness of the Achilles tendon. The subjects of their study were 33 young (24 ± 2 years old) and 67 older (75 ± 3 years old) healthy adults. They found that the older adults had 17% lower Achilles tendon stiffness than the younger adults (p < 0.01).
In addition, the older adults had 32% lower Young’s modulus than their younger counterparts (p < 0.001). Some have hypothesized that the decrease in tendon stiffness that occurs with age may require larger fascicle strains to generate the same amount of force for isometric contractions. These larger fascicle strains could cause the sarcomeres to function at a less optimal region of their force length relationship. This could then affect the ability of muscle to produce strong contractions. Csapo, Malis, Hodgson, and Sinha (2014) tested this hypothesis using velocity-encoded phase-contrast magnetic resonance imaging of the Achilles tendon and gastrocnemius medialis muscle. The subjects of their study were six young (26.1± 2.3 years old) and six older (76.7 ± 8.3 years old) healthy adult women.
The researchers found that the fascicle lengths of the gastrocnemius medialis muscle were shorter in the older women by 10-20% which was significant. Additionally, they found that the older women had significantly lower Achilles tendon stiffness when compared to the younger women (p < 0.05). However, their results showed that the fascicle lengths during submaximal isometric contraction for the two age groups were almost equal.
The results of this study were unexpected, and they indicate that Achilles tendon stiffness does not affect the fascicle strain. Csapo, Malis, Hodgson, and Sinha (2014) explained their results through the concept of fascicle slack. Fascicle slack refers to the delay in the lengthening of fascicles when compared to the muscle tendon unit (Herbert et al., 2011). Thus, it is possible that the younger women had higher fascicle slack. Thus, the greater tendon stiffness and higher fascicle slack of the younger women would oppose each other and result in similar levels of fascicle shortening as was observed in this study.
Decreased Fascicle Length and Sarcomere Length
Muscle performance deteriorates with aging due to changes in muscle quality and declines in the cross-sectional area of skeletal muscle as mentioned previously. However, there are additional changes that occur in muscle such as changes in the length of fascicles and sarcomeres which also contribute to the age-related declines in muscle performance. Crooks, Power, and Herzog (2015) investigated the age associated changes in fascicle length, sarcomere length and series sarcomeres. Their muscle of interest was the medial gastrocnemius. They found that older rats had 14% shorter muscle fascicles when compared to the younger rats. As a result, there was 10% less sarcomeres in series.
Other studies conducted on humans have also found that older adults have shorter fascicle lengths. Crooks, Power, and Herzog (2015) determined that the older rats had 4% shorter sarcomere lengths (Crooks, Power, & Herzog, 2015). It is important to consider that shorter fascicle lengths could shift the force length relationship such that sarcomeres would produce lower force because they are not at their optimal length. However, since the force length relationship is determined by the sarcomere length and not fascicle length this would only occur if reductions in the fascicle length were unaccompanied by reductions in the number of sarcomeres in series.
So, if the number of sarcomeres remained the same or did not lower with respect to the reduction in fascicle lengths, then we would find that the sarcomeres would need to be shorter to fit in the new shorter fascicle length. If this occurred, then each sarcomere would now be outside of their optimal length and as a result producing less force. This is shown visually in figure 3.
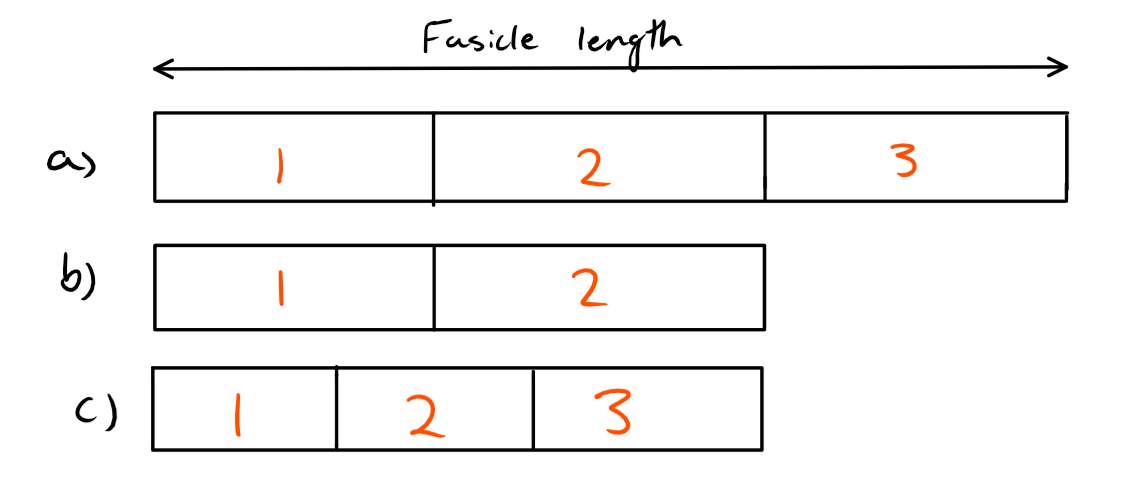
Figure 3: Three different scenarios are shown in this figure. The part marked a) shows the original fascicle length with 3 sarcomeres at their optimal length. Part b) shows that the fascicle length has been reduced, but so has the number of sarcomeres so that each sarcomere is still at their optimal length. Part c) shows that the fascicle length has been reduced, but the number of sarcomeres has not been reduced with respect to the fascicle length. As a result, each sarcomere is shorter then their optimal length and we would expect the force-length relationship to be altered.
In this study, they found that the sarcomere length was shorter and therefore we would expect the force length relationship to be shifted to the left, since the sarcomeres are not at their optimal length.
Changes in the Force Velocity Relationship with Aging
With aging there is a reduction in the ability of muscle to produce force for given velocities of contraction. The decline is highest for concentric contractions. This means that with age you are no longer able to produce high amounts of force for a given velocity of concentric contraction. Hortobagyi et al. (1995) studied the effect of aging on muscle strength during isometric, concentric, and eccentric contractions. The participants of their study were healthy adult men between the ages of 18-80 years and healthy adult women between the ages of 20-70 years. Their muscle of interest was the quadriceps muscle. They found that eccentric strength was mainly preserved with age and observed only a 9N decline per decade. However concentric and isometric forces declined about 30N per decade which was significant (p < 0.05).
Pousson, Lepers & Van Hoecke (2001) conducted a similar study investigating the changes in the torque-angular velocity relationship with age and found similar results. They determined that eccentric strength was preserved with age while concentric and isometric strength declined significantly. Hortobagyi et al, (1995) suggested that the preservation of eccentric strength may be a result of older adults having less sarcomere instability. The removal of weak sarcomeres would cause this reduced sarcomere instability. Additionally, they explained that eccentric cross-bridge cycling may be altered with age and thus allow higher force production with eccentric contractions.
Porter, Vandervoort & Kramer (1997) investigated the explanations that Hartobagyi et al. (1995) made. They measured the plantar flexors and dorsiflexors eccentric and concentric strength for 16 older (67 ± 4 years old) and 16 younger (27 ± 4 years old) adult women. They found that the rate of torque development was significantly lower for older women when compared to younger women for both muscle groups. The researchers suggested that this was a result of slower cross-bridge cycling and loss of type II muscle fibers. Although, slower cross-bridge cycling is disadvantageous for high velocity concentric contractions, it is critical in producing high forces in eccentric contractions. Therefore, Porter, Vandervoort & Kramer (1997) concluded that the preservation of eccentric strength with age is a result of a slower rate of torque development which is in turn caused by slower cross-bridge cycling.
More recently, titin has been found to have a significant role in eccentric contractions and some have even called for a three-filament model of contraction to account for titin (Herzog, 2014). Since titin is so important for eccentric contractions, it is possible that with aging this filament is preserved or altered to a lesser degree. This would then result in the preservation of eccentric strength which is often observed in older adults.
Since power and force are linked by the equation, Power = Force * Velocity, we would expect power to decline as force production decreases with age. Unsurprisingly, maximum power is reduced with age and the contraction velocity at maximum power is also reduced. However, the reduction in maximum power is far greater than the reduction in muscle strength that is observed with aging (Toji & Kaneko, 2007). The reason for this difference is because power also depends on velocity which is also decreasing with age.
Thom, Morse, Birch & Narici (2007) studied the changes in the maximum velocity of shortening (Vmax) that occur with age. Their subjects were 9 older (74.7 ± 4 years old) and 15 younger men (25.3 ± 4.5 years old). They discovered that the older men’s gastrocnemius muscle had a 38.2% lower Vmax when compared to the younger men (p < 0.001). This difference in Vmax was partly explained by the differences in fascicle length between the young and old age groups. When they took in to account the differences in fascicle length, the difference in Vmax was reduced to 15.9%. The age associated decline in power and force is summarized in figure 4. Thus, power is reduced more significantly than force because the velocity of shortening is also declining.
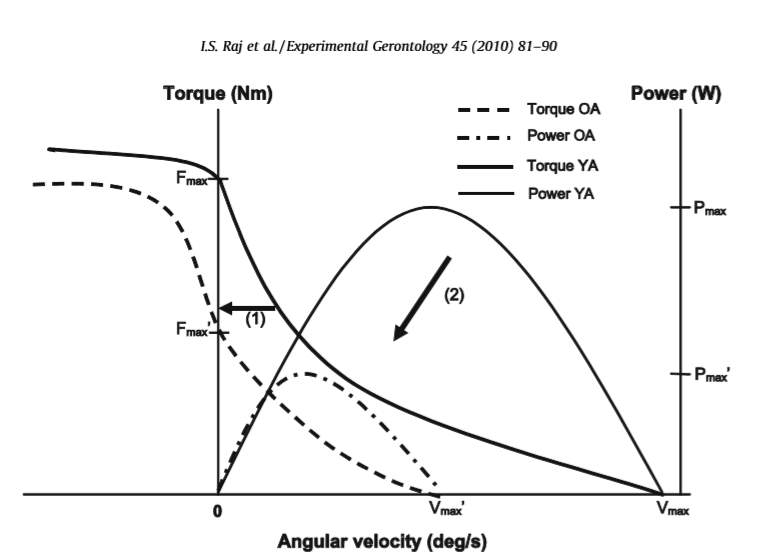
Figure 4: The comparison of power and force-velocity relationships for young adults(YA) and older adults (OA). Older adults have a lower maximum force (Fmax), velocity of shortening (Vmax), and maximum power (Pmax). The preservation of eccentric strength is also shown.
Many studies have investigated the functional implications of the age-associated declines in the force-velocity and power-velocity relationships. Function in tasks such as climbing stairs, walking, and sit to stand are often studied as they provide insights into how the older adults’ daily lives are affected. The general finding is that there is a correlation between power and functional performance and a correlation between muscle strength and functional performance. However, the correlation between power and functional performance is stronger than that of muscular strength.
Suzuki, Bean & Fielding (2001) studied the relationship between power and strength on performance in functional tasks such as stair-climb time, repeated chair rise time, and maximal gait velocity. The participants of this study were 34 older women (75.4 ± 5.1 years old). A dynamometer was utilized to measure the peak torque and power of the plantar flexors and dorsiflexors. They found that there was a significant correlation between dorsiflexor peak power and stair climb time (r = 0.49, p < 0.003) and chair rise time (r = 0.50, p < 0.002). Additionally, there was a significant relationship between plantar flexor strength and maximal gait velocity (r = 0.47, p < 0.005). Their results supported the notion that muscle power is more important than muscle strength in terms of performance in functional tasks.
Architectural Changes in Aged Muscle
The age associated deterioration in muscle performance is influenced by a variety of factors which work together. A particularly important factor is architectural changes in the muscle which then affects other determinants of muscle performance such as the force-length and force-velocity relationship. Two of the most critical architectural factors affecting muscle performance are fascicle length and pennation angle.
Narici, Maganaris, Reeves, and Capodaglio (2003) studied the effect of aging on muscle architecture. They utilized ultrasonography to measure the fascicle length and pennation angle of the gastrocnemius muscle for 14 young (27-42 years) and 16 older (70-81 years) healthy older adults. They ensured that both age groups were physically active so that any differences that were elucidated would be due to age and not physical inactivity which often accompanies aging. The older adults had 10.2% (p < 0.01) shorter fascicle lengths when compared to the young age group. We have mentioned previously that shorter fascicle lengths can cause the force-length relationship to shift to the left. However, we must also consider that if the number of sarcomeres is decreased such that each sarcomere is still at their optimal length to fit the new shorter fascicle lengths, then the velocity of muscle shortening is decreased. This would cause a decline in overall power since power is directly proportional to velocity. But, the strain rate would still be the same even with shorter fascicle lengths. Therefore, volume specific power will be conserved, if we only consider shorter fascicle lengths.
But, as we have discussed before aging is accompanied by a reduction in muscle quality and a fast to slow fiber type shift, so volume specific power is also decreased, but the shorter fascicle lengths does not contribute. To summarize, shorter fascicle lengths cause a reduced range of motion and decrease the force and/or power generating capacity of the muscle.
The force developed by muscle is dependent on the cosine of the pennation angle. Therefore, lower pennation angles are more favourable as the muscle fibers will be contracting with the line of action of the tendon and more force will be transmitted to the tendon which will then be transmitted to bone.
In the study by Narici, Maganaris, Reeves & Capodaglio, (2003) they found that older adults had a 13.2% (p< 0.01) lower pennation angle. This occurs because with age the size of muscle fibers decreases and as a result there is less packing of contractile fibers. This lower pennation angle is advantageous as it will lead to higher force production. However, it is important to note that although the difference in pennation between the two age groups was significant, it was not huge. There was approximately a 4 degrees difference in pennation between the two age groups which leads to a 3% higher force production in the older adults. However, when we consider the declines in muscle quality and cross-sectional area and many of the other factors mentioned previously we will find that those force reductions massively overshadow this slight force increase.
Pennate muscles are significant because of their role in variable gearing which influences muscle performance (Holt, Danos, Roberts & Azizi, 2016). In pennate muscles, the fibers rotate during contraction to alter the ratio of muscle shortening velocity to fiber shortening velocity. For instance, if the fibers undergo a large rotation during contraction, the velocity of muscle shortening will be higher than the velocity of fiber shortening. Therefore, the fibers will produce more force because they are acting at a lower velocity of shortening. However, since we have a high pennation angle, less of the force will be acting in the line of action of the tendon. If the fibers undergo a minor rotation during contraction, then the velocity of muscle shortening will be more similar to that of the fiber shortening velocity. This lower pennation angle means that the fibers will be acting in the line of action of the tendon. But, there will be less force production from the fibers because they are shortening at a higher velocity.
It has been hypothesized that variable gearing occurs due to the interplay between connective tissue and contractile tissue (Holt, Danos, Roberts & Azizi, 2016). During muscle contraction, the volume of the muscle is maintained and therefore as muscle shortens the width and/or thickness of muscle must increase. Contractile tissue provides resistance to increases in muscle thickness whereas connective tissue resists changes in muscle width such as bulging. So, when fibers produce a low amount of force, there is low resistance to increases in muscle thickness. As a result, muscle thickness increases, and the fibers rotate to higher pennation angles which means high gear ratios. When fibers produce a high amount of force there is high resistance to increases in muscle thickness. Thus, muscle width increases and there is less fiber rotation resulting in low gear ratios.
Holt, Danos, Roberts, and Azizi (2016) hypothesized that variable gearing would be diminished in older adults’ muscles because of the changes in connective and contractile tissue that accompany aging. They studied the gastrocnemius muscle of young (5-9 months) and old (33-34 months) rats. They determined that the older rats had a significantly higher fiber bundle modulus (p < 0.05) and transverse aponeurosis modulus (p < 0.05) when compared to the younger rats. In accordance with other papers they found that pennation angle decreased with age (Narici, Maganaris, Reeves & Capodaglio, 2003). They found that there was a significant relationship between relative force and gear ratio in young rats (p < 0.001), but no significant relationship in older rats. In addition, they showed that their findings held true even when the relationship between absolute force and gear ratio was determined for the range of forces generated by old muscle.
This demonstrated that the loss of variable gearing was not just because older muscles have a lower force-generating capacity. Figure 5 shows that as absolute force increased, the gear ratio decreased in the young rats. This relationship was not observed in the older rats. The loss of variable gearing means that old muscle functions at high gear ratios since it is unable to reduce the gear ratio. At high gear ratios there is a large rotation of fibers resulting in large pennation angles and as a result less of the force is acting in the line of action of the tendon. Thus, the loss of variable gearing is another factor that contributes to the reduced stress generating capacity of older muscle.
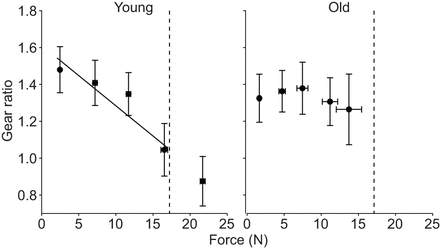
Figure 5: The relationship between gearing ratio and absolute force for young and old rats
Conclusion
The deterioration in muscle performance associated with age is a multifactorial issue. The reduction in the cross-sectional area of skeletal muscle is often cited as a major contributor to the reduced muscle strength and power observed in older adults. However, when researchers account for the reduced cross-sectional area of muscle, the reductions in strength and power persist. This indicates that the quality of muscle has also been reduced. The quality of muscle is compromised because of a fast-to-slow fiber type shift and the infiltration of fat into muscle which reduces myofibrillar content. Furthermore, there are changes in the muscle architecture such as shortening of fascicle lengths and reductions in the pennation angle of pennate muscles.
These changes then reduce the maximum velocity of shortening and cause the force length relationship to shift to the left. Additionally, with age there is a reduction in the force generating capacity of muscle during concentric and isometric contractions. But, eccentric strength is preserved. Moreover, with age there is a loss in the ability of muscle to undergo variable gearing. All these factors contribute to the decline in muscle performance that accompanies aging. This decline in performance has a significant negative impact on grip strength, walking, climbing stairs and sit to stand.
These activities are an important part of daily living and affect the quality of life of older adults. Additionally, these losses in muscle performance make it more difficult for older adults to participate in physical activity which further promotes loss of muscle mass and therefore strength and power. Thus, it is crucial to use our knowledge about the factors causing the deterioration in muscle performance to establish therapies that will impede these declines.
References
Crooks, S., Power, G.A., Herzog, W. (2015). Aging is Associated with Reductions in Fascicle Length, Sarcomere Length and Serial Sarcomeres, 5, 4–8.Ciciliot, S., Rossi, A., Dyar, K., Blaauw, B., & Schiaffino, S. (2013). Muscle type and fiber type specificity in muscle wasting. The International Journal Of Biochemistry & Cell Biology, 45(10), 2191-2199. http://dx.doi.org/10.1016/j.biocel.2013.05.016
Csapo, R., Malis, V., Hodgson, J., & Sinha, S. (2014). Age-related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. Journal Of Applied Physiology, 116(8), 961-969. http://dx.doi.org/10.1152/japplphysiol.01337.2013
Delmonico MJ,Harris TB,Visser M,Park SW,ConroyMB, VelasquezMieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH (2009) Longitudinal study of muscle strength, quality, andadipose tissue infiltration.Am J Clin Nutr 90, 1579–1585
Dreyer, H., Blanco, C., Sattler, F., Schroeder, E., & Wiswell, R. (2006). Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle & Nerve, 33(2), 242-253. http://dx.doi.org/10.1002/mus.20461
Herbert, R., Clarke, J., Kwah, L., Diong, J., Martin, J., & Clarke, E. et al. (2011). In vivopassive mechanical behaviour of muscle fascicles and tendons in human gastrocnemius muscle-tendon units. The Journal Of Physiology, 589(21), 5257-5267. http://dx.doi.org/10.1113/jphysiol.2011.212175
Herzog, W. (2014). The role of titin in eccentric muscle contraction. Journal Of Experimental Biology, 217(16), 2825-2833. http://dx.doi.org/10.1242/jeb.099127
Hodgson, J., Chi, S., Yang, J., Chen, J., Edgerton, V., & Sinha, S. (2012). Finite element modeling of passive material influence on the deformation and force output of skeletal muscle. Journal Of The Mechanical Behavior Of Biomedical Materials, 9, 163-183. http://dx.doi.org/10.1016/j.jmbbm.2012.01.010
Holt, N., Danos, N., Roberts, T., & Azizi, E. (2016). Stuck in gear: age-related loss of variable gearing in skeletal muscle. The Journal Of Experimental Biology, 219(7), 998-1003. http://dx.doi.org/10.1242/jeb.133009
Hortobagyi, T., Zheng, D., Weidner, M., Lambert, N., Westbrook, S., & Houmard, J. (1995). The Influence of Aging on Muscle Strength and Muscle Fiber Characteristics With Special Reference to Eccentric Strength. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 50A(6), B399-B406. http://dx.doi.org/10.1093/gerona/50a.6.b399
Jankowski, C. (2008). Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Yearbook Of Sports Medicine, 2008, 276-277. http://dx.doi.org/10.1016/s0162-0908(08)79074-3
Janssen, I., Heymsfield, S., Wang, Z., & Ross, R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal Of Applied Physiology, 89(1), 81-88. http://dx.doi.org/10.1152/jappl.2000.89.1.81
Kent-Braun, J., Ng, A., & Young, K. (2000). Skeletal muscle contractile and noncontractile components in young and older women and men. Journal Of Applied Physiology, 88(2), 662-668. http://dx.doi.org/10.1152/jappl.2000.88.2.662
Maden-Wilkinson, T., McPhee, J., Jones, D., & Degens, H. (2015). Age-Related Loss of Muscle Mass, Strength, and Power and Their Association with Mobility in Recreationally-Active Older Adults in the United Kingdom. Journal Of Aging And Physical Activity, 23(3), 352-360. http://dx.doi.org/10.1123/japa.2013-0219
Marcell, T. (2003). Review Article: Sarcopenia: Causes, Consequences, and Preventions. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 58(10), M911-M916. http://dx.doi.org/10.1093/gerona/58.10.m911
Miljkovic N, Lim JY, Miljkovic I, Frontera WR. Aging of Skeletal Muscle Fibers. Ann Rehabil Med. 2015 Apr;39(2):155-162. https://doi.org/10.5535/arm.2015.39.2.155
Narici, M., Maganaris, C., Reeves, N., & Capodaglio, P. (2003). Effect of aging on human muscle architecture. Journal Of Applied Physiology, 95(6), 2229-2234. http://dx.doi.org/10.1152/japplphysiol.00433.2003
Newman, A., Haggerty, C., Goodpaster, B., Harris, T., Kritchevsky, S., & Nevitt, M. et al. (2003). Strength and Muscle Quality in a Well-Functioning Cohort of Older Adults: The Health, Aging and Body Composition Study. Journal Of The American Geriatrics Society, 51(3), 323-330. http://dx.doi.org/10.1046/j.1532-5415.2003.51105.x
Nilwik, R., Snijders, T., Leenders, M., Groen, B., van Kranenburg, J., Verdijk, L., & van Loon, L. (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Experimental Gerontology, 48(5), 492-498. http://dx.doi.org/10.1016/j.exger.2013.02.012
Population Projections for Canada, Provinces and Territories: Highlights. (2018). Statcan.gc.ca. Retrieved 20 March 2018, from https://www.statcan.gc.ca/pub/91-520-x/2010001/aftertoc-aprestdm1-eng.htm
Porter, M., Vandervoort, A., & Kramer, J. (1997). Eccentric Peak Torque of the Plantar and Dorsiflexors Is Maintained in Older Women. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 52A(2), B125-B131. http://dx.doi.org/10.1093/gerona/52a.2.b125
Pousson, M., Lepers, R., & Van Hoecke, J. (2001). Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Experimental Gerontology, 36(10), 1687-1698. http://dx.doi.org/10.1016/s0531-5565(01)00143-7
Proctor, D., Sinning, W., & Sieck, G. (1995). OXIDATIVE CAPACITY OF HUMAN MUSCLE FIBER TYPES: EFFECTS OF AGE AND TRAINING STATUS. Medicine & Science In Sports & Exercise, 27(Supplement), S43. http://dx.doi.org/10.1249/00005768-199505001-00247
Rahemi, H., Nigam, N., & Wakeling, J. (2015). The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. Journal Of The Royal Society Interface, 12(109), 20150365. http://dx.doi.org/10.1098/rsif.2015.0365
Renault, V., Thorne, L., Eriksson, P., Butler-Browne, G., & Mouly, V. (2002). Regenerative potential of human skeletal muscle during aging. Aging Cell, 1(2), 132-139. http://dx.doi.org/10.1046/j.1474-9728.2002.00017.x
Siparsky, P. N., Kirkendall, D. T., & Garrett, W. E. (2014). Muscle Changes in Aging: Understanding Sarcopenia. Sports Health, 6(1), 36–40. http://doi.org/10.1177/1941738113502296
Stenroth, L., Peltonen, J., Cronin, N., Sipilä, S., & Finni, T. (2012). Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. Journal Of Applied Physiology, 113(10), 1537-1544. http://dx.doi.org/10.1152/japplphysiol.00782.2012
Suzuki, T., Bean, J., & Fielding, R. (2001). Muscle Power of the Ankle Flexors Predicts Functional Performance in Community-Dwelling Older Women. Journal Of The American Geriatrics Society, 49(9), 1161-1167. http://dx.doi.org/10.1046/j.1532-5415.2001.49232.x
The Daily — Age and sex, and type of dwelling data: Key results from the 2016 Census. (2018). Statcan.gc.ca. Retrieved 21 March 2018, from http://www.statcan.gc.ca/daily-quotidien/170503/dq170503a-eng.htm
Therkelsen, K., Pedley, A., Hoffmann, U., Fox, C., & Murabito, J. (2016). Intramuscular fat and physical performance at the Framingham Heart Study. AGE, 38(2). http://dx.doi.org/10.1007/s11357-016-9893-2
Thom, J., Morse, C., Birch, K., & Narici, M. (2007). Influence of muscle architecture on the torque and power–velocity characteristics of young and elderly men. European Journal Of Applied Physiology, 100(5), 613-619. http://dx.doi.org/10.1007/s00421-007-0481-0
Toji, H., & Kaneko, M. (2007). Effects of Aging on Force, Velocity, and Power in the Elbow Flexors of Males. Journal Of PHYSIOLOGICAL ANTHROPOLOGY, 26(6), 587-592. http://dx.doi.org/10.2114/jpa2.26.587
Verdijk, L., Gleeson, B., Jonkers, R., Meijer, K., Savelberg, H., Dendale, P., & van Loon, L. (2009). Skeletal Muscle Hypertrophy Following Resistance Training Is Accompanied by a Fiber Type-Specific Increase in Satellite Cell Content in Elderly Men. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 64A(3), 332-339. http://dx.doi.org/10.1093/gerona/gln050
Verdijk, L., Koopman, R., Schaart, G., Meijer, K., Savelberg, H., & van Loon, L. (2007). Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. American Journal Of Physiology-Endocrinology And Metabolism, 292(1), E151-E157. http://dx.doi.org/10.1152/ajpendo.00278.2006
Visser, M., Goodpaster, B., Kritchevsky, S., Newman, A., Nevitt, M., & Rubin, S. et al. (2005). Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 60(3), 324-333. http://dx.doi.org/10.1093/gerona/60.3.324
Welle, S., Totterman, S., & Thornton, C. (1996). Effect of Age on Muscle Hypertrophy Induced by Resistance Training. The Journals Of Gerontology Series A: Biological Sciences And Medical Sciences, 51A(6), M270-M275. http://dx.doi.org/10.1093/gerona/51a.6.m270
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




